Monitoring of Antibiotic Resistance
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotic Prescribing And Antibiotic Stewardship
To get the most out of a newly implemented steward-ship programme (or a change to an existing one) it is necessary to have the means by which to measure its effect.
MONITORING OF ANTIBIOTIC RESISTANCE
To get the most out of a newly implemented steward-ship programme (or a
change to an existing one) it is necessary to have the means by which to
measure its effect. It is, therefore, necessary to plan in advance what
parameters will be measured and what will be the baseline data against which the
changes will be judged. Some of the parameters might include, but are not restricted
to:
• Antibiotic consumption and costs, both in total and by specific drug class
• Costs associated with prescribing potentially toxic antibiotics, e.g.
gentamicin and vancomycin blood level monitoring
• Rates of resistance to specific antibiotics by problem pathogens
• Pharmacy interventions to advise on inappropriate antibiotic use
• The incidence of hospital-acquired infections.
One of the problems that has dogged reviewers trying to assess the
extent of the benefits of stewardship programmes is that in many of the cases
reported in the medical literature multiple changes to an established programme
have been introduced together, or they have overlapped in time so that
evaluating the contribution of each change has been difficult. It is,
therefore, worthwhile deciding in advance when a new policy or practice will be
implemented and when its effect will be assessed. The input of information
technology specialists and hospital epidemiologists to a stewardship management
team becomes important since they, together, can decide how the data will be
recorded and analysed to best effect.
The more sophisticated antibiotic
control and information systems do not simply record data on antibiotic
consumption, cost and resistance, but are capable of relating infection control
data to antibiotic use and would be expected to draw attention to situations
where a change in use of a particular antibiotic was associated ith increasing
isolation of a particular pathogen. Such n association does not, of course,
mean that one caused the other, but it does
raise staff awareness of that potential. Computer-assisted surveillance of
hospital-acquired infection (HAI) has been shown, in some cases, to be more
effective than manual monitoring and reporting; as long ago as 1986 one study
reported that 90% of antibiotic-resistant HAIs were detected by computer compared
to 76% manually. There is also the potential to improve antibiotic prescribing
by minimizing the risk of adverse effects when information systems provide
patient-specific warnings on allergies, immune and renal functions and the
potential for interaction with the patient’s other drugs.
It is not surprising, perhaps, that
there are marked differences in antibiotic resistance patterns from one country
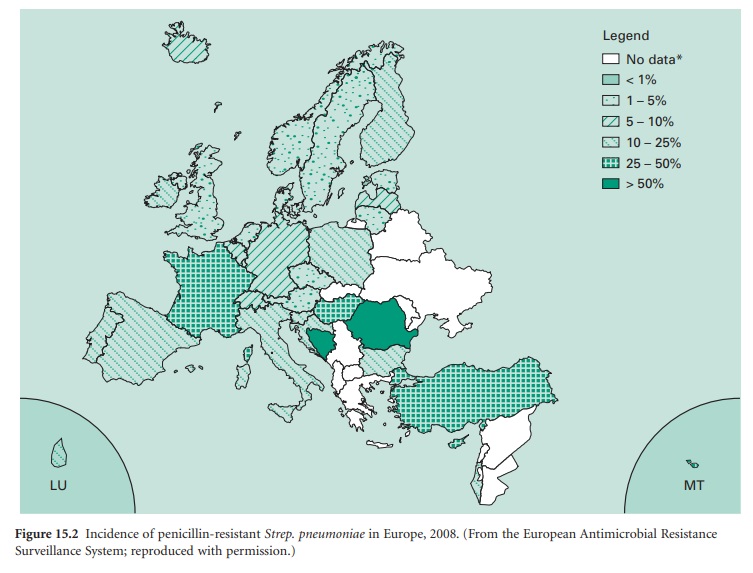
to another. This is illustrated in Figure 15.2,
which shows that penicillin resistance in Streptococcus pneumoniae can
vary from less than 5% in some European countries to more than 50% in others.
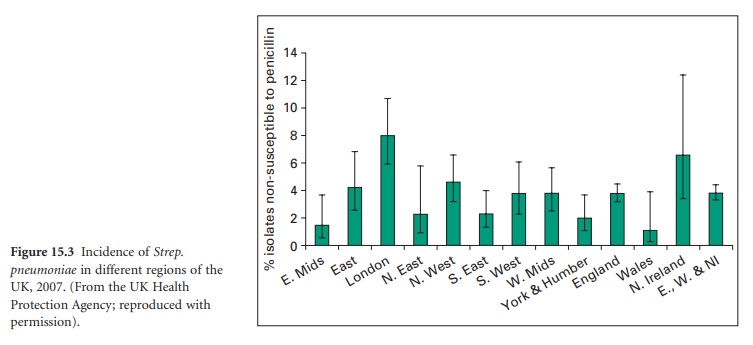
Smaller, but nevertheless significant, variations may also arise between
different regions of a single country; Figure 15.3 shows
the corresponding Strep. pneumoniae data
for the UK from the national Heath Protection Agency. However, data on local
resistance patterns are of paramount importance and well-structured monitoring
programmes should be capable of identifying unforeseen consequences of changes
in antibiotic use such as that arising when a preapproval policy for
cephalosporins was introduced in a New York hospital in an attempt to control
cephalosporin resistance in Klebsiella species.
The policy did achieve a 71% reduction in ceftazidime-resistant Klebsiella isolated in intensive care units, but
monitoring revealed a concomitant rise in imipenem use and a 69% increase in
imipenem-resistant Ps. aeruginosa that
was attributed largely to the preapproval policy.
Related Topics
