Morphine analogues
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Narcotic Analgesics
Narcotic Analgesics - Morphine analogues - Synthesis and Drug Profile - a. Morphine (Duraphine) b. Levorphanol (Levo-Dromoran) c. Buprenorphine (Tidigesic, Norphin, Buprinor) d. Etorphine - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose
SYNTHESIS AND DRUG PROFILE
Morphine analogues
a. Morphine (Duraphine)
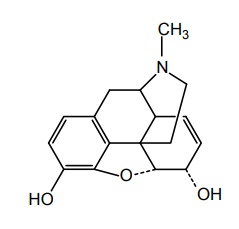
Metabolism of morphine: Morphine is conjugated by hepatic enzyme at phenolic (3-OH) position to from 3-glucuronide metabolite. Glucuronidation of morphine also leads to N-demethylation to normorphine, which has decreased opioid activity and it undergoes N and O conjucation and excreted. Compounds with N-alkyl groups larger than methyl get N-dealkylated as a major route of inactivation.
Properties and uses: It exists as a white or almost white crystalline powder or colourless, silky needles or cubical masses, efflorescent in a dry atmosphere. Soluble in water, slightly soluble in ethanol, and insoluble in toluene. It used as an opioid receptor agonist and analgesic.
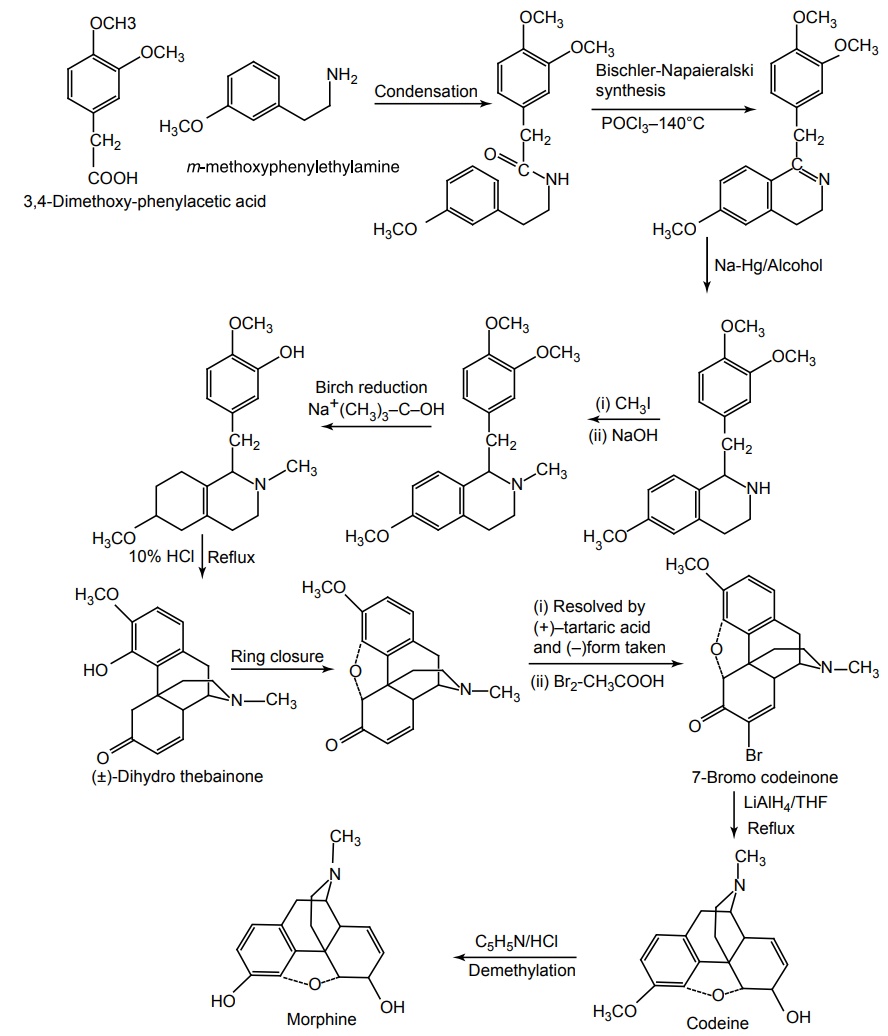
Synthesis
Assay: Dissolve the sample in a mixture of 0.01M hydrochloric acid and ethanol. Perform potentiometric titration, using 0.1M sodium hydroxide.
Storage: It should be stored in well-closed airtight containers and protected from light.
Dose: The usual, adult, oral dose is 10 to 30 mg 6 times/day.
Dosage forms: Chloroform and Morphine tincture I.P., B.P., Morphine suppositories I.P., B.P., Morphine sulphate injection I.P.
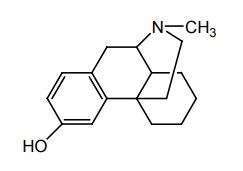
b. Levorphanol (Levo-Dromoran)
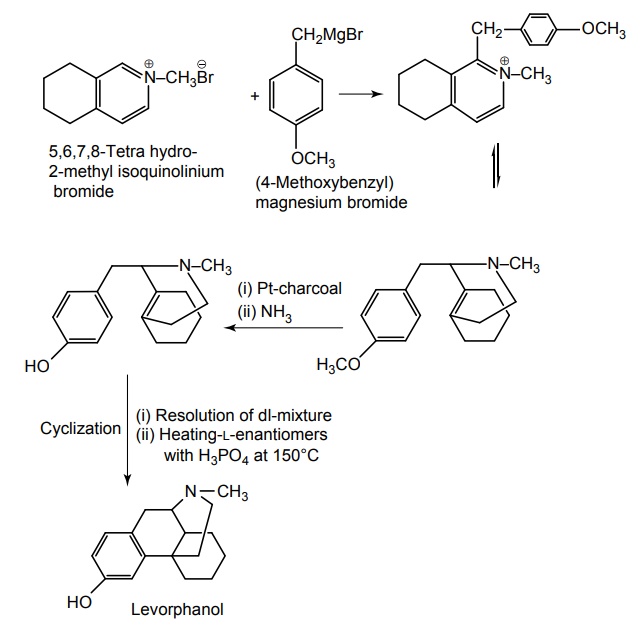
Synthesis
Properties and uses: It exists as white, odourless, crystalline powder, soluble in water and alcohol, insoluble in chloroform and ether. It is a potent narcotic analgesic having actions and structure similar to that of morphine. It is used effectively for the management of both moderate and severe pain. The d-isomer shows antitussive property .
Dose: By oral for severe pain, 1.5 to 4.5 mg 1 or 2 times daily; by S.C, I.M., usual single dose is 2 to 4 mg.
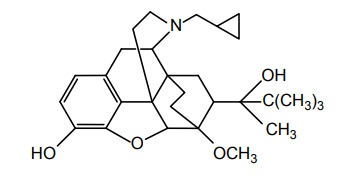
c. Buprenorphine (Tidigesic, Norphin, Buprinor)
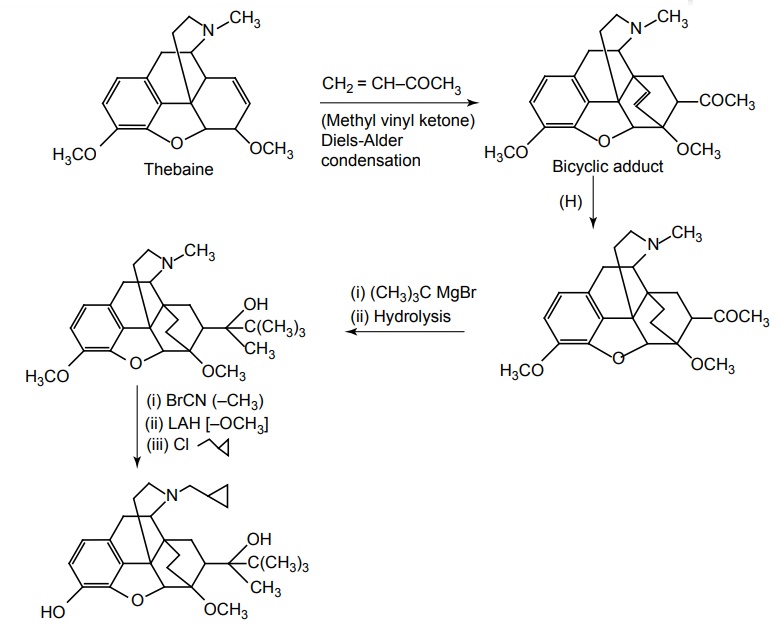
Synthesis
Properties and uses: It exists as a white or almost white crystalline powder. Insoluble in cyclohexane, sparingly soluble in water, freely soluble in methanol, and alcohol. Buprenorphine is a more complex molecule than morphine, which would interact with the opioid receptor (analgesic) and because of its complex structure it would not interact with other receptors that produce side effects, and used as potent analgesic.
Assay: Dissolve the substance in anhydrous acetic acid and add acetic anhydride to this. Titrate against
0.1 M perchloric acid and determine the end point potentiometrically.
Storage: It should be stored in well-closed airtight container and protected from light.
Dose: The usual dose is 0.3 mg I.M. every 6 h.
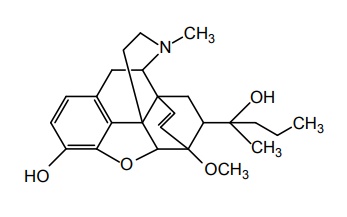
d. Etorphine
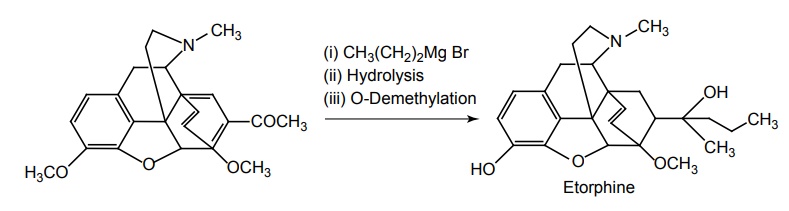
Synthesis
Properties and uses: It is a white or almost white microcrystalline powder, very slightly soluble in chloroform, insoluble in ether, sparingly soluble in water and in ethanol. Etorphine is thousand times more potent than morphine, which could be interpreted as having a better or a tighter fit to receptors. It is used primarily in veterinary medicine to immobilize large animals.
Preparations: Etorphine and Acepromazine injection B.P., Etorphine and Levomepromazine injection B.P.
Assay: Assayed by nonaqueous titration, to a solution of a sample, add mercury (II) acetate solution and titrate against 0.1M perchloric acid, using crystal violet solution as indicator.
Storage: It should be stored in well-closed airtight containers and protected from light.
Related Topics
