SAR of Morphine
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Narcotic Analgesics
SAR of Morphine was studied by 1. Modification of alicyclic ring 2. Modification of aromatic ring 3. Modification of 3o Nitrogen
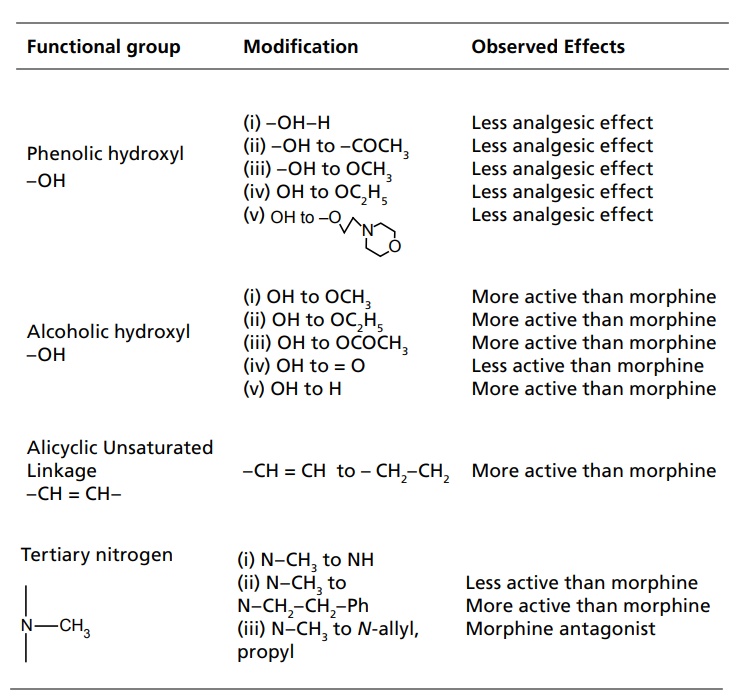
SAR of Morphine SAR of Morphine was studied by Modification of alicyclic ring Modification of aromatic ring Modification of 3o Nitrogen The alcoholic hydroxyl group at C-6 when methylated, esterified, oxydized, removed, or replaced by halogen analgesic activity as well as toxicity of the compound increased. The reduction of C-6 keto group to C-6 β hydroxyl in oxymorphone gives Nalbupine, it shows antagonistic action of μ receptors. The saturation of the double bond at C-7 position gives more potent compound. Examples, Dihydro morphine and Dihydro codeine. The 14 β hydroxyl group generally enhances μ agonistic properties and decreases antitussive activity. However, activity varies with the overall substitution on the structure. Bridging of C-6 and C-14 through ethylene linkage gives potent derivatives. Reaction of thebaine with dienophile (i.e diel’s alder reaction) results in 6, 14 endo etheno tetrahydro thebaine derivatives, which are commonly called ‘oripavines’. Some oripavines are extremely potent μ agonist, for example, Etorphine and Buprenorphine are the best known. These derivatives are about thousand times more potent than morphine as μ agonist. An aromatic phenyl ring is essential for activity. Modification on phenolic hydroxyl group decreases the activity. Any other substitution on phenyl ring diminishes activity. A tertiary amine is usually necessary for good opioid activity. The size of the N substitution can dictate the compounds potency and its agonists and its reverse antagonistic property. The N-methyl substitution is having good agonistic property, when increased the size of the substitution by 3–5 carbons results in antagonistic activity. Still larger substitutent on N returns agonistic property of opioids, for example, N-phenyl ethyl substitution is ten times more potent than N-methyl groups. N-allyl and N-cylo alkyl group leads to narcotic antagonistic property. Removal of 3,4 epoxide bridge in morphine structure result in the compound that is refered to as morphinans. The morphinans are prepared synthetically. As the synthetic procedure yielded compound is a racemic mixture, only levo isomer possesses opioid activity while the dextro isomer has useful antitussive activity, for example, Levorphanol and Butorphanol. Levorphanol is a more potent analgesic than morphine. Summarized SAR of Morphine Analogues is given below: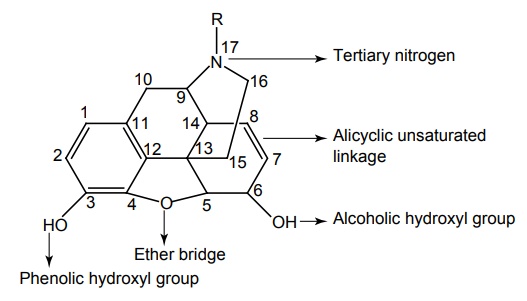
1. Modification on alicyclic ring
2. Modification on phenyl ring
3. Modification of 3° nitrogen
4. Epoxide Bridge
Related Topics
