NSAIDs and Prostaglandin (PG) Synthesis Inhibition
| Home | | Pharmacology |Chapter: Essential pharmacology : Nonsteroidal Anti-inflammatory Drugs And Antipyreticanalgesics
In 1971 Vane and coworkers made the landmark observation that aspirin and some NSAIDs blocked PG generation. This is now considered to be the major mechanism of action of NSAIDs.
NSAIDS AND PROSTAGLANDIN (PG) SYNTHESIS INHIBITION
In 1971 Vane and
coworkers made the landmark observation that aspirin and some NSAIDs blocked PG
generation. This is now considered to be the major mechanism of action of
NSAIDs. Prostaglandins, prostacyclin (PG I2) and thromboxane A2
(TXA2) are produced from arachidonic acid by the enzyme
cyclooxygenase which
exists in a constitutive (COX1) and an inducible (COX2) isoforms; the former
serves physiological ‘house keeping’ functions, while the latter, normally
present in minute quantities, is induced by cytokines and other signal molecules
at the site of inflammation → generation of PGs locally which mediate many
of the inflammatory changes. However, COX2 is constitutively present at some
sites in brain and in juxtaglomerular cells: may serve physiological role at
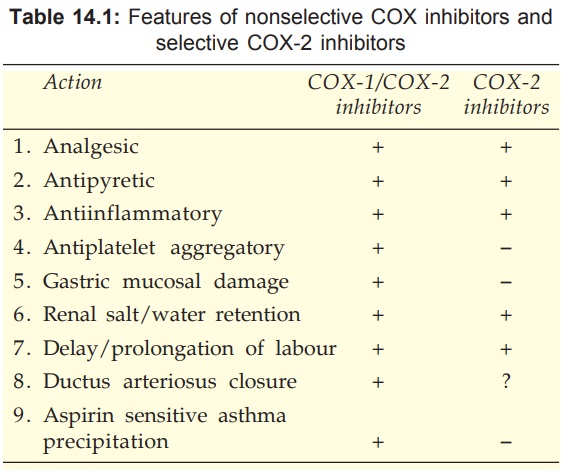
these sites. Most NSAIDs inhibit COX1 and COX2 nonselectively, but now some
selective COX2 inhibitors have been produced. Features of nonselective COX1/COX2
inhibitors (traditional NSAIDs) and selective COX2 inhibitors are compared in
Table 14.1
Aspirin
inhibits COX irreversibly by acetylating one of its serine residues; return of
COX activity depends on synthesis of fresh enzyme.
Beneficial actions due to PG synthesis inhibition
· Analgesia: prevention of pain nerve ending
sensitization
·
Antipyresis
·
Anti-inflammatory
·
Antithrombotic
·
Closure of ductus arteriosus in newborn
Other NSAIDs are competitive and reversible inhibitors of COX,
return of activity depends on their dissociation from the enzyme which in turn
is governed by the pharmacokinetic characteristics of the compound.
Analgesia PGs induce hyperalgesia by affecting the
transducing property of free nerve endings—stimuli that normally do not elicit
pain are able to do so. NSAIDs do not affect the tenderness induced by direct
application of PGs, but block the pain sensitizing mechanism induced by
bradykinin, TNFα, interleukins (ILs) and other algesic substances. They are,
therefore, more effective against inflammation associated pain.
Antipyresis NSAIDs reduce body temperature in fever, but do not cause hypothermia in normothermic
individuals. Fever during infection is produced through the generation of
pyrogens including, ILs, TNFα , interferons which induce PGE2 production
in hypothalamus—raise its temperature set point. NSAIDs block the action of
pyrogens but not that of PGE2 injected into the hypothalamus. The
isoform present at this site appears to be COX2 (possibly COX3 also). However,
fever can occur through non-PG mediated mechanisms as well.
Shared toxicities due
to PG synthesis inhibition
1. Gastric mucosal damage
2. Bleeding: inhibition
of platelet function
3. Limitation of renal
blood flow : Na+ and water retention
4. Delay/prolongation of
labour
5. Asthma and anaphylactoid
reactions in susceptible individuals
Anti-inflammatory The most important
mechanism of anti-inflammatory action of NSAIDs is considered to be inhibition
of PG synthesis at the site of injury. The anti-inflammatory potency of different
compounds roughly corresponds with their potency to inhibit COX. However, nimesulide
is a potent anti-inflammatory but relatively weak COX inhibitor. PGs are only
one of the mediators of inflammation; inhibition of COX does not depress the
production of other mediators like LTs, PAF, cytokines, etc. Inflammation is
the result of concerted participation of a large number of vasoactive,
chemotactic and proliferative factors at different stages, and there are many
targets for anti-inflammatory action.
Activated endothelial cells express adhesion molecules (ECAM 1, ICAM1) on their surface and play a key role in directing circulating leucocytes to the site of inflammation (chemotaxis). Similarly, inflammatory cells express selectins and integrins. Certain NSAIDs may act by additional mechanisms including inhibition of expression/ activity of some of these molecules and generation of superoxide/other free radicals. Growth factors like GMCSF, IL6 and lymphocyte transformation factors may also be affected. Stabilization of leucocyte lysosomal membrane and antagonism of certain actions of kinins may be contributing to NSAID action.
Dysmenorrhoea Involvement of PGs in
dysmenorrhoea has been clearly demonstrated: level of PGs in menstrual flow,
endometrial biopsy and that of PGF2α metabolite in
circulation are raised in dysmenorrhoeic women. Intermittent ischaemia of the
myometrium is probably responsible for menstrual cramps. NSAIDs lower uterine
PG levels—afford excellent relief in 60–70% and partial relief in the
remaining. Ancillary symptoms of headache, muscle ache and nausea are also
relieved. Excess flow may be normalized.
Antiplatelet aggregatory NSAIDs inhibit synthesis
of both proaggregatory (TXA2) and antiaggregatory (PGI2) prostanoids,
but effect on platelet TXA2 (COX1 generated) predominates therapeutic
doses of most NSAIDs inhibit platelet aggregation: bleeding time is prolonged.
Aspirin is highly active; acetylates platelet COX irreversibly in the portal
circulation before it is deacetylated by first pass metabolism in liver. Small
doses are therefore able to exert antithrombotic effect for several days. Risk
of surgical bleeding is enhanced.
Ductus
arteriosus closure During foetal circulation ductus arteriosus is
kept patent by local elaboration of PGE2 and PGI2. Unknown
mechanisms switch off this synthesis at birth and the ductus closes. When this
fails to occur, small doses of indomethacin or aspirin bring about closure in
majority of cases within a few hours by inhibiting PG production.
Administration of NSAIDs in late pregnancy has been found to promote premature
closure of ductus in some cases. Prescribing of NSAIDs near term should be
avoided.
Parturition
Sudden spurt of PG synthesis by uterus probably
triggers labour and facilitates its progression. Accordingly, NSAIDs have the
potential to delay and retard labour. However, labour can occur in the absence
of PGs.
Gastric
mucosal damage Gastric pain, mucosal erosion/ulceration and
blood loss are produced by all NSAIDs to varying extents: relative gastric
toxicity is a major consideration in the choice of NSAIDs. Inhibition of COX1
mediated synthesis of gastroprotective PGs (PGE2, PGI2)
is clearly involved, though local action inducing back diffusion of H+ ions in
gastric mucosa also plays a role. Deficiency of PGs reduces mucus and HCO3¯
secrection, tends to enhance acid secretion and may promote mucosal ischaemia.
Thus, NSAIDs enhance aggressive factors and contain defensive factors in
gastric mucosa—are ulcerogenic. Paracetamol, a very weak inhibitor of COX is
practically free of gastric toxicity and selective COX2 inhibitors are safer.
Stable PG analogues (misoprostol) administered concurrently with NSAIDs
antagonise their gastric toxicity.
Renal effects Conditions leading to
hypovolaemia, decreased renal perfusion and Na+ loss induce renal PG synthesis
which brings about intrarenal adjustments by promoting vasodilatation,
inhibiting tubular Cl¯ reabsorption (Na+ and water accompany) and opposing ADH
action.
NSAIDs
produce renal effects by at least 3 mechanisms:
·
COX1 dependent impairment of renal blood flow
and reduction of g.f.r. → can worsen renal insufficiency.
·
Juxtaglomerular COX2 (probably COX1 also)
dependent Na+ and water retention.
·
Ability to cause papillary necrosis on
habitual intake.
Renal
effects of NSAIDs are not marked in normal individuals, but become significant
in those with CHF, hypovolaemia, hepatic cirrhosis, renal disease and in
patients receiving diuretics or antihypertensives: Na+ retention and edema can
occur; diuretic and antihypertensive drug effects are blunted.
Involvement
of PG synthesis inhibition in analgesic nephropathy is uncertain.
Anaphylactoid reactions Aspirin precipitates asthma, angioneurotic swellings, urticaria or rhinitis in certain susceptible individuals. These subjects react similarly to chemically diverse NSAIDs, ruling out immunological basis for the reaction. Inhibition of COX with consequent diversion of arachidonic acid to LTs and other products of lipoxygenase pathway may be involved, but there is no proof.
Related Topics
