Oestrogen and Venous Thromboembolism
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: The Cardiovascular Spectrum of Adverse Drug Reactions
Identification and confirmation of the adverse effects of postmenopausal hormone therapy illustrate yet a third approach to risk assessment. Oestrogen has been used to treat menopausal symptoms since 1933 when emmenin was introduced.
OESTROGEN AND VENOUS
THROMBOEMBOLISM
Identification
and confirmation of the adverse effects of postmenopausal hormone therapy
illustrate yet a third approach to risk assessment. Oestrogen has been used to
treat menopausal symptoms since 1933 when emmenin was introduced. Premarin, a
more easily manufactured oestrogen, was approved in 1942 (CDER, 1997; FDA,
2003). By the 1960s, 12% of women in the United States were using
post-menopausal oestrogen therapy, a proportion that increased steadily. The
National Prescription Audit and National Disease and Therapeutic Index
databases tracked annual hormone therapy prescriptions rising from 58 million
in 1995 to 90 million in 1999; prescriptions then remained stable through June
2002 (Hersh, Stefanick and Stafford, 2004). Analysis of data from a large
cohort study in the United States showed that 45% of postmenopausal women used
oestrogen for at least a month and more than 20% used it for 5 or more years,
either alone or in combination with progestin (Brett and Madans, 1997).
These
rates have been shown to differ based on a woman’s hysterectomy status; in a
study by Keating and colleagues in the early 1990s, current post-menopausal
hormone use was 58.7% among women with prior hysterectomy compared to 19.6%
among women with intact uteri (Keating, Manassiev and Stevenson, 1999). Most
women started to take ther-apy shortly after menopause; median duration of use
was 3 years (mean 6.6 years). Postmenopausal hormone use demonstrated a secular
trend; only 19% of women born before 1904 ever used postmenopausal hormones,
compared to 63% of women born between 1945 and 1954 (Brett and Madans, 1997).
In
1992, the American College of Physicians recom-mended hormone therapy for
postmenopausal women who either had hysterectomy or were at risk of coronary
heart disease (American College of Physi-cians, 1992). It quickly became
standard medical practice to prescribe exogenous oestrogens, either alone or in
combination with progestin, for most menopausal women, with the expectation that
most, if not all of these women, would benefit from treatment. Initially, most
women received unopposed oestro-gen regardless of their hysterectomy status.
After the National Heart, Lung and Blood Institute-funded Post-menopausal
Oestrogen/Progestin Interventions (PEPI) trial reported an increased risk of
endometrial hyper-plasia when women with intact uteri were treated with
unopposed oestrogen in 1995, most women with intact uteri were switched to
combination oestrogen– progestin therapy (The Writing Group for the PEPI Trial,
1996). Indeed, when the Women’s Health Initia-tive was being planned in the
early 1990s, there was debate about the ethics of withholding post-menopausal
hormone therapy from the women who would be randomized to placebo.
A
cloud was introduced to that climate of enthu-siasm for oestrogen in the 1960s
when an apparent increased risk of venous thromboembolism (VTE), that is, deep
venous thrombosis and pulmonary embolism, was associated with oral
contraceptive use (Royal College of General Practitioners, 1967; Vessey and
Doll, 1968, 1969; Jick et al., 1995;
Spitzer, 1997).
The
relationship between VTE and exogenous oestro-gen was explored in several small
case-control and cohort studies in the 1970s (BCDSP, 1974; Nachtigall et al., 1979; Petitti et al., 1979). In these analyses, VTE was more common in women taking
oral contra-ceptives, but the relationship with postmenopausal hormone therapy
was less clear. These epidemiologic studies were followed by large randomized,
controlled trials.
The
PEPI was a 3-year randomized, placebo-controlled trial in 875 postmenopausal
women comparing the effects of several postmenopausal hormone regimens on
cardiovascular disease risk factors. The cohort was healthy and relatively
young; consequently, only ten VTE cases were identified among women on active
hormone therapy and none on placebo during the 3-year follow-up (The Writing
Group for the PEPI Trial, 1995). The rate of VTE in women taking conjugated
oestrogens (0.625 mg daily) alone was twice that of women taking any of three
oestrogen plus progestin regimens (Table 41.2), but the overall number of cases
was small.
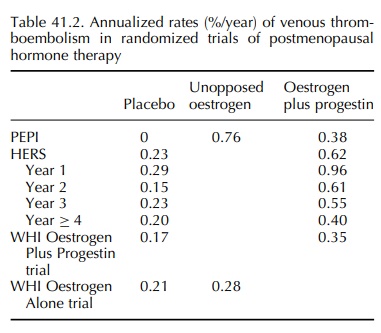
The
Heart & Oestrogen/Progestin Replacement Study (HERS) randomized 2763 women
with hormone therapy increased VTE risk (relative hazard 2.7, 95% confidence
interval 1.4–5.0); the rela-tive hazard for deep venous thrombosis was 2.8 (95%
confidence interval 1.3–6.0) and for pulmonary embolism was 2.8 (95% confidence
interval 0.9–8.7) with oestrogen plus progestin.
The
Women’s Health Initiative (WHI) includes two randomized, placebo-controlled
hormone trials, one with unopposed conjugated oestrogens (0.625 mg daily) in 10
739 women with prior hysterectomy, and the other with conjugated oestrogens
0.625 mg plus medroxyprogesterone acetate 2.5 mg daily in 16 608 women with
intact uteri. In the trial of unopposed oestrogen, the hazard ratio for VTE was
1.33 (95% confidence interval 0.99–1.79) (WHI Writing Group, 2004). In the
trial of combination oestrogen plus progestin, the hazard ratio for VTE was
2.06 (95% confidence interval 1.57–2.70) (Cushman et al., 2004). In these predominantly healthy women, the annualized
rates of VTE were lower than in HERS (Table 41.2), but the studies demonstrated
a similar pattern of risk by year of treatment. In the WHI Oestrogen Plus
Progestin trial, the yearly hazard ratios were 4.01 in year 1, 1.97 in year 2,
1.74 in year 3, 1.70 in year 4, 2.90 in year 5 and 1.04 in year 6 or later.
In
a Bayesian meta-analysis which included PEPI and HERS, but not the WHI, the
overall relative risk of VTE with postmenopausal hormone therapy was 2.14 (95%
credible interval 1.64–2.81) (Miller, Chan and Nelson, 2002). This
meta-analysis also supported the observation in HERS that the greatest risk for
thromboembolic events with oestrogen was during the first year (relative risk
3.49, 95% credible interval 2.33–5.59).
The
labels for oestrogen formulations have been repeatedly updated to reflect new
findings, including the risk of VTE. A major change was made in 1998, when a
warning was added stating,
In some epidemiological studies,
women on oestro-gen replacement therapy, given alone or in combi-nation with a
progestin, have been reported to have an increased risk of thrombophlebitis,
and/or throm-boembolic disease, although the evidence is conflict-ing In some
epidemiological studies, women on oestrogen replacement therapy, given alone or
in combination with a progestin, have been reported to have an increased risk
of thrombophlebitis, and/or thromboembolic disease, although the evidence is
conflicting (FDA, 1998).
Following
release of the WHI results in 2002, a black box statement pertaining to
cardiovascular risks was added to the label for oestrogen. This state-ment
read, ‘The women’s health initiative (WHI) reported increased risks of
myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and
deep vein thrombosis in postmenopausal women during 5 years of treatment with
conjugated equine oestrogens (0.625 mg) combined with medroxypro-gesterone
acetate (2.5 mg) relative to placebo.’ The warning goes on to state that the
FDA assumes these findings will hold for all HRT formulations contain-ing
oestrogen and suggests that HRT drugs should be used in the lowest doses
necessary for the shortest duration possible (FDA, 2003).
The
WHI experience altered the way the medical community, lay public and regulatory
agencies viewed the entire issue of drug safety. Awareness of the need for long
term randomized studies of commonly accepted therapies has been enhanced, along
with the importance of ‘real world’ follow-up studies of drugs – many of which
were approved long before current pharmacovigilance guidelines.
Related Topics
