Altered Pharmacokinetics in the Elderly
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Drugs and the Elderly
Elderly patients may also develop drug-related problems even when their medication is confined to a single agent or non-interacting multiple agents.
ALTERED PHARMACOKINETICS IN THE
ELDERLY
Elderly
patients may also develop drug-related problems even when their medication is
confined to a single agent or non-interacting multiple agents. This may relate
to pharmacokinetic and pharmaco-dynamic changes associated with ageing. Such
age-related physiological changes may alter the way in which the body handles
medication, leading to changes in drug disposition in the elderly patient.
ABSORPTION
Following
oral administration, most drugs dissolve in the stomach. Little absorption
takes place here because of the small surface area and low pH, which means that
drugs which are weak bases are in an ionised state. Absorption primarily takes
place in the small intestine because of the large surface area and high pH,
which favours the unionised state of most drugs. With increasing age, many
changes occur in the gastrointestinal tract which should make the rate and
extent of absorption less predictable, including a reduction in acid secretion
in the stomach, decreased gastric emptying, diminished splanchnic blood flow and
decreased gastrointestinal mobility (Geokas and Haverback, 1969; Evans et al., 1981; Greenblatt et al., 1982; Goldberg and Roberts,
1983; Montamat et al., 1989;
Woodhouse, 1994). However, in practice, few drugs have significantly delayed
rates of absorption (Greenblatt et al.,
1982; Woodhouse, 1994). This is probably because potentially rate-limiting
factors in the small intestine (such as surface area and lumi-nal pH) are not
altered to a critical degree. A recent review by Cusack (2004) concludes that
stud-ies performed in the last decade assessing extent of absorption by
comparing area under the curve (AUC) after oral and IV administration and rate
of absorp-tion using Tmax corroborate the prevailing opinion that ageing does
not affect absorption to a significant degree.
Once drugs are absorbed from the gut, they enter the portal
circulation and must pass through the liver before entering the systemic
circulation. The bioavail-ability of most polar or water-soluble drugs is not
affected by age because they are not highly extracted by the liver. For many
lipophilic drugs, this first pass through the liver is accompanied by
pronounced (sometimes over 90%) extraction with only 5%–10% of the dose
reaching the systemic circulation. It is clear that a small change in hepatic
function may result in a large increase in bioavailability in those drugs which
undergo a high presystemic first-pass metabolism (Montamat et al., 1989; Woodhouse, 1994). For exam-ple, decreased presystemic
extraction in the elderly may lead to increases in the bioavailability of
propra-nolol (Castleden and George, 1979) and nifedipine (Robertson et al., 1988), but usually not to a
clinically significant extent. The changes may be more marked, however, in the
frail and hospitalised elderly (Wood-house, 1994). Bioavailability may also be
regulated by presystemic extraction by small bowel cytochrome P-450 3A4 and by
the extrusive action of P-glycoprotein on the surface of cells in the small
bowel. Limited data do not support the effect of ageing on either process
(Cusack, 2004).
DISTRIBUTION
Following
absorption of a drug, the extent to which it is distributed within the body
depends on body composition, plasma protein binding and blood flow.
Body Composition
With
age, there is a decrease in lean body mass and body water and a corresponding
increase in adipose tissue in relation to total body weight (Edelman and
Leibman, 1959; Forbes and Reina, 1970; Novak, 1972). Adipose tissue increases
from about 18% to 36% in men and from 33% to 45% in women (Novak, 1972).
Therefore, the distribution of lipid-insoluble drugs such as paracetamol
(Divoll et al., 1982) or ethanol
(Vestal et al., 1977) may decrease in
the elderly. This means that plasma concentra-tions per unit dose are higher.
Lipid-soluble drugs such as diazepam are more widely distributed in the elderly
and may have prolonged action and a ‘hang-over’ effect because of the longer
elimination half-life (Macklon et al.,
1980).
Protein Binding
Serum albumin levels decline with age, but in healthy elderly
people this change is minimal. More marked reductions appear to relate to
disease, immobility and poor nutrition rather than age itself (MacLennan et al., 1977; Campion, de Labrey and
Glynn, 1988). This reduction may
result in a decrease in the bind-ing capacity of weakly acidic drugs such as
salicy-lates and phenytoin (Wallace and Verbeeck, 1987). Measurement of the
plasma-free drug concentration (which will be increased under these
circumstances) may be a better guide to the dose requirements than the total
plasma concentration, particularly if the ther-apeutic ratio is low (Grandison
and Boudinot, 2000). However, a raised free fraction will also result in an
increased clearance allowing a new steady state to be achieved with regular
dosing. Total plasma drug concentrations may then be lower, but free-drug
concentrations will remain the same as these are deter-mined by hepatic or
renal clearance of free drug. On the other hand, -l-acid glycoprotein increases
with age, and basic drugs such as lignocaine display increased protein binding
in elderly patients (Cusack et al.,
1980).
Metabolism
Although
some drugs are eliminated directly by the kidneys, many undergo metabolism in
the liver first. Clearance of drugs by the liver depends on the activ-ity of
the enzymes responsible for biotransformation and on blood flow, which
determines the rate of delivery of the drug to the liver. For drugs that are
metabolised relatively slowly by the liver (those with low intrinsic
clearance), clearance is proportional to the rate of hepatic metabolism
(Woodhouse, 1994). Hepatic mass decreases with age by 25%–35%, so the
metabolism of such drugs may be reduced (Wood-house and James, 1990).
The
metabolic pathways involved in the biotrans-formation of drugs may be divided
into two phases (Williams, 1967). Phase 1 reactions comprise oxida-tive,
reductive or hydrolytic processes which render the compound less lipophilic but
can be fully or partly active. Products of phase 1 may then undergo phase 2
reactions which involve glucuronidation, sulphation or acetylation. The
resulting conjugates are much more polar than the parent compound, usually have
little or no pharmacological activity and are gener-ally excreted in the urine.
Phase 1 oxidative drug metabolism may be reduced in the elderly (O’Malley et al., 1971), but phase 2 reactions are
generally thought not to be altered,
at least in fit elderly patients. However, in the frail elderly, in those who
have suffered injury or have undergone surgery, enzyme activity may be
significantly depressed, resulting in higher blood concentrations and an
increased risk of adverse reactions (Woodhouse, 1994). In particular, a
reduction in plasma aspirin esterase activity, paraceta-mol conjugation and
metabolism of metoclopromide and theophylline have been reported in frail
elderly patients (Wynne et al., 1990,
1993; Groen et al., 1993; Israel et al., 1993).
Metabolism
of many drugs, such as the benzodi-azepines, may involve phase 1 followed by
phase 2 reactions. Diazepam undergoes oxidative (phase 1) metabolism and its
elimination is prolonged in the elderly (Belantuono et al., 1980). It is also partly converted to an active metabolite,
desmethyl-diazepam, which has a half-life of up to 220 hours in elderly people.
However, other benzodiazepines, such as lorazepam, undergo conjugation
reactions in the liver, and their metabolism is unaltered by age. These
compounds which do not give rise to active compounds may therefore be safer for
elderly people to use than the other benzodiazepines (Williams and Lowenthal,
1992).
Age
may not be the only factor that affects drug metabolism. Cigarette smoking,
alcohol intake, dietary considerations, drugs, illnesses and caffeine intake
may be equally important (Vestal et al.,
1975; Montamat et al., 1989). In
addition, hepatic blood flow rather than microsomal enzyme activity is the
major determinant of total clearance of many drugs which have a very rapid rate
of metabolism and consequently high extraction rates across the liver. Hepatic
blood flow is 35% lower in healthy people over 65 years of age than in young
people (Wynne et al., 1989).
Reductions in systemic clear-ance of drugs with high hepatic extraction ratios
(including presystemic clearance) have been reported in elderly people. Such
drugs include propranolol (Castleden and George, 1979), clomethiazole (Nation et al., 1976) and morphine (Baillie et al., 1989), and the reduced clearance is compatible with a decline in liver blood
flow.
RENAL EXCRETION
Most
polar drugs or polar drug metabolites are elimi-nated by the kidney after
filtration at the glomerulus. In addition, drugs such as the -lactam
antibiotics are actively secreted in the proximal tubules. As part of normal
ageing, both renal functional capac-ity and renal reserve diminish. The
structural changes include a decrease in renal weight, thickening of the
intrarenal vascular intima, a reduction in the number of glomeruli with
increased sclerosis within those remaining and infiltration by chronic
inflammatory cells and fibrosis in the stroma (Muhlberg and Platt, 1999).
Altered renal tubular function may also lead to impaired handling of water,
sodium and glucose in old age. There is a steady decline in the glomeru-lar
filtration rate by approximately 8 ml/minute per decade (Rowe et al., 1976). By the age of 70,
there-fore, a person may have a 40%–50% reduction in renal function (even in
the absence of overt renal disease).
Drug
elimination may be reduced even in patients with normal serum creatinine
concentrations because creatinine production decreases with age. Many drugs
which are dependent on the kidney for elimina-tion will accumulate to toxic
levels if given in the usual doses to
elderly people. Examples include digoxin (Smith, 1973), atenolol (McAinsh,
1977) and amiloride (George, 1980). In addition, reduced clearance of active
metabolites of certain drugs may increase the risk of toxicity particularly in
very elderly patients. One example is morphine-6-glucuronide, the active
metabolite of morphine (McQuay et al.,
1990). Furthermore, many drugs themselves adversely affect renal function in
the elderly, for example aminoglyco-sides, diuretics, NSAIDs and
angiotensin-converting enzyme (ACE) inhibitors. In this way, age-dependent
changes in renal function are responsible for altered pharmacokinetics in the
elderly, but in many cases, the kidneys are the target for the ADRs produced by
these changes (Muhlberg and Platt, 1999).
As drug elimination is correlated to creatinine clear-ance, estimating the creatinine clearance may be help-ful in deciding whether a dose reduction is necessary. A useful method that may be used at the bedside is the Cockcroft formula (Cockcroft and Gault, 1976):
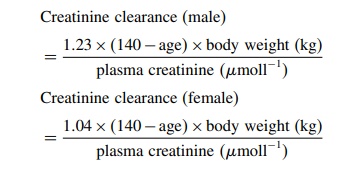
The
diagnostic value of age and creatinine clear-ance (calculated by the Cockcroft
formula) for the prediction of potentially toxic drug plasma levels has been
reviewed by Muhlberg and Platt (1999). They found that 256 geriatric patients
with many differ-ent illnesses have been studied in 17 pharmacokinetic studies
with 17 different drugs, including angiotensin-converting enzyme inhibitors,
NSAIDs, antibiotics, beta-blockers, bronchodilators and benzodiazepines.
Mathematical simulation and pharmacokinetic meth-ods were used to determine
whether a dose reduction was necessary in elderly patients with a reduced
crea-tinine clearance determined by the Cockcroft formula. For most drugs
studied, elevated plasma levels at steady state could be correctly predicted
when the creatinine clearance was < 40 ml/min, particularly when age was
taken into account, suggesting that a dose reduction was necessary. This
confirms the usefulness of the Cockcroft formula for clinical use in elderly
patients taking drugs which are eliminated in the kidney and which are toxic at
higher plasma concentrations. When using drugs with a low ther-apeutic ratio,
estimation of the creatinine clearance helps determine the initial dose but,
when possible, should be supplemented by therapeutic drug monitor-ing (Cusack,
2004).
Related Topics
