Other Databases in Europe for the Analytic Evaluation of Drug Effects
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Other Databases in Europe for the Analytic Evaluation of Drug Effects
In the ranking of abstracts that were based on auto-mated databases, the first positions were taken by Denmark, The Netherlands and Italy, which were consistent in 2000 and 2005.
Other Databases in
Europe for the Analytic Evaluation of Drug Effects
INTRODUCTION
A systematic review of the abstracts presented at the 16th
and 21st International Conference on Phar-macoepidemiology in 2000 and 2005,
respectively, showed that the majority (53% and 51%) of submit-ted European
pharmacoepidemiological studies were conducted by means of automated general
practitioner (GP), pharmacy or insurance data (Table 29.1). Little has changed
between 2000 and 2005. The United Kingdom ranked highest in number in 2000,
basi-cally because of the wide use of the General Prac-tice Research Database
(GPRD) within the United Kingdom itself. In 2005, the majority of GPRD-based
abstracts comes from outside the United King-dom. Twenty-five abstracts were
based on data from the GPRD, eight from the United States, nine from the United
Kingdom, five from the Netherlands and three from Spain. The UK databases will
not be further discussed in this chapter as they are covered elsewhere in this
book.
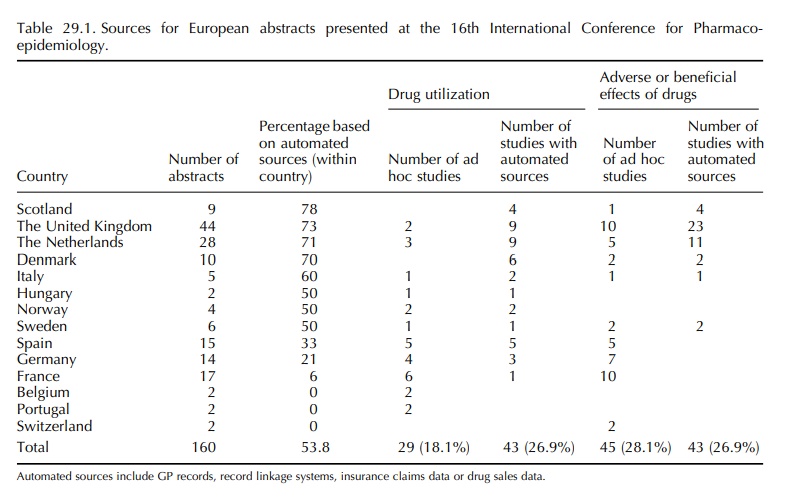
In
the ranking of abstracts that were based on auto-mated databases, the first
positions were taken by Denmark, The Netherlands and Italy, which were
consistent in 2000 and 2005. Spain ranked high in 2005 because of the use of
the GPRD by the Spanish Center for Pharmacoepidemiology (CEIFE). A Span-ish
general practice database is being established in collaboration with CEIFE
(www.bifap.es), but valida-tion processes are on its way.
The
Netherlands is well known for the PHARMO Record Linkage System (www.pharmo.nl)
and the Integrated Primary Care Information (IPCI) (www.ipci.nl) GPRD. Other
dispensing databases (Interaction) are occurring, but little information is
available on them so far. In addition, there are fixed cohort studies that are
linked to pharmacy data (e.g. Rotterdam study) and have proven useful for
phar-macoepidemiological research, but ad hoc studies fall outside the scope of
this chapter. Pharmacoepidemi-ology in the Netherlands is strong because of the
availability of various academic training and doctoral programmes, the
organization of health care and the availability of high-quality data (Leufkens
and Urquhart, 2005).
Denmark
is well known for its regional and national dispensing databases that can be
linked to other national registries such as the cancer, mortality and
hospitalization registry. Initially only Jutland and Funen county had
prescription databases, but since 2003 the national prescription database can
be used and linked to other registries at Statistics Denmark. This creates the
unique possibility to study an entire country and provides a strong backbone
for pharma-coepidemiologic research in Denmark.
The Italian story is quite different. In 2000, the Ital-ian studies were mostly based on claims databases that are compiled for National Health Service (NHS) payments (regional or local). Nowadays access to regional databases is complicated because of privacy legislations. Local databases are sometimes used for pharmacoepidemiological studies, but not all have the same structure and quality, combination of these databases at a local level may provide opportuni-ties in the future. Since 2000, a general practice database (former name Health Search) and paedia-tricians’ medical record database (PEDIANET) have gained importance in the field of pharmacoepidemi-ology. Both of these databases will be discussed.
The
results from the abstract review underline that national differences in the
organization and reim-bursement of health care have a major impact on the
possibility to conduct pharmacoepidemiologic research (Leufkens and Urquhart,
2005). The coun-tries with the highest numbers of abstracts are flour-ishing
because of specific features of the systems for health care delivery and the
presence of (phar-maco)epidemiologists. Italy has many databases that are
suitable for pharmacoepidemiological research, but the output is limited so far
because of the scarcity of academic (pharmaco)epidemiology programmes.
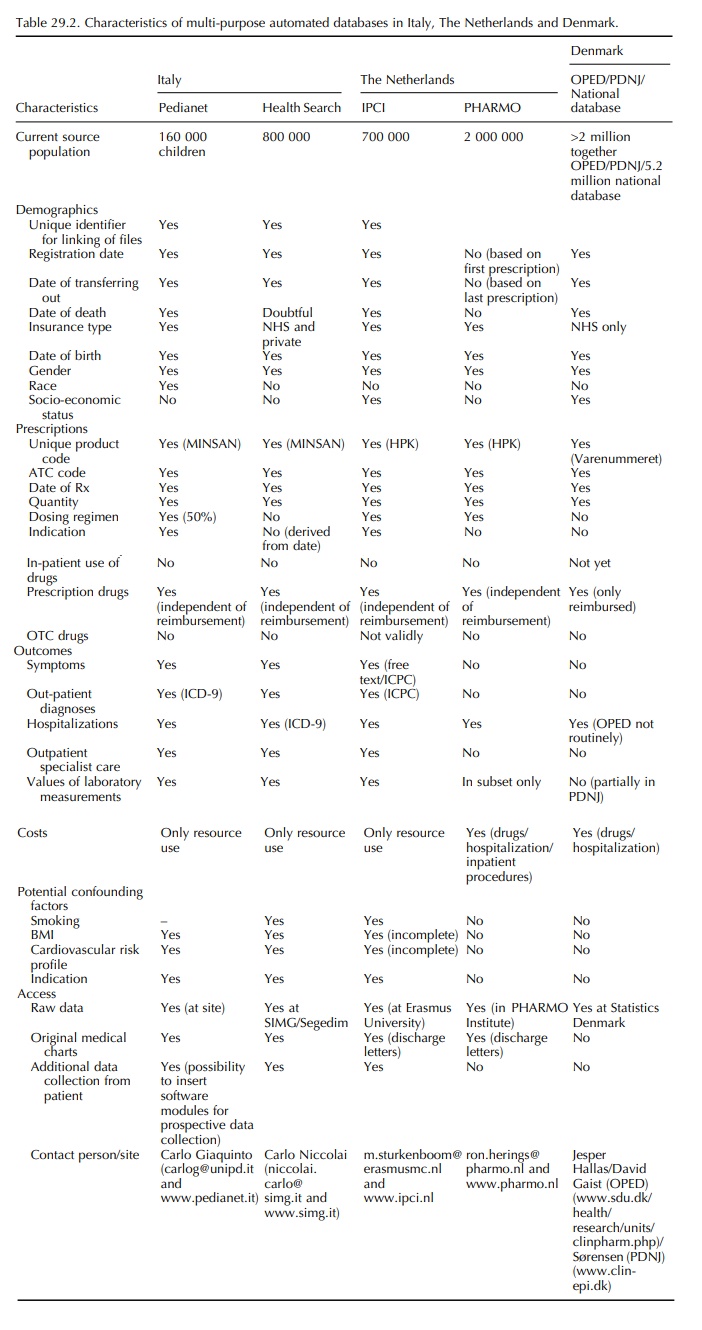
This chapter describes general practice and record linkage
databases from the Netherlands, Italy and Denmark, which have yielded
peer-reviewed phar-macoepidemiological papers during the period 1990 and 2005.
Since the quality of databases depends on the health care systems they are
embedded in, a summary of the major health care characteristics will be
provided for each of these countries. Table 29.2 provides a systematic overview
of the characteristics of the databases.
Related Topics
