Polyene Antibiotics
| Home | | Pharmacology |Chapter: Essential pharmacology : Antifungal Drugs
The name polyene is derived from their highly double-bonded structure. Amphotericin B is described as the prototype.
POLYENE ANTIBIOTICS
The name polyene is derived from their highly
double-bonded structure. Amphotericin B is described as the prototype.
Amphotericin B (AMB)
It is obtained from Streptomyces nodosus.
Chemistry And Mechanism Of Action
The polyenes possess a
macrocyclic ring, one side of which has several conjugated double bonds and is
highly lipophilic, while the other side is hydrophilic with many OH groups. A
polar amino-sugar and a carboxylic acid group are present at one end in some.
They are all insoluble in water and unstable in aqueous medium.
The polyenes have high affinity for ergosterol present in fungal
cell membrane: combine with it, get inserted into the membrane and several
polyene molecules together orient themselves in such a way as to form a
‘micropore’. The hydrophilic side forms the interior of the pore through which
ions, amino acids and other water-soluble substances move out. The micropore is
stabilized by membrane sterols which fill up the spaces between the AMB
molecules on the lipophilic side—constituting the outer surface of the pore.
Thus, cell permeability is markedly increased.
Cholesterol, present in host cell membranes, closely resembles
ergosterol; the polyenes bind to it as well, though with lesser affinity. Thus,
the selectivity of action of polyenes is low, and AMB is one of the most toxic
systemically used antibiotics, though it is the least toxic polyene. Bacteria
do not have sterols and are unaffected by polyenes. It has been found that AMB
enhances immunity in animals, and this may aid immunocompromised individuals in
handling fungal infection.
Antifungal Spectrum
AMB is active against
a wide range of yeasts
and fungi—Candida albicans, Histoplasma capsulatum, Cryptococcus
neoformans, Blastomyces dermatitidis, Coccidioides immitis, Torulopsis,
Rhodotorula, Aspergillus, Sporothrix, etc. Dermatophytes are inhibited in
vitro, but concentrations of AMB attained in infected skin are low and
ineffective. It is fungicidal at high and static at low concentrations.
Resistance to AMB during therapy has been rarely noted among Candida in a selected group of
leucopenic cancer patients, but it is not a problem in the clinical use of the
drug.
AMB is also active on various species of Leishmania.
Pharmacokinetics
AMB is not absorbed
orally; it can be given orally
for intestinal candidiasis without systemic toxicity. Administered i.v. as a
suspension made with the help of deoxycholate (DOC), it gets widely distributed
in the body, but penetration in CSF is poor. It binds to sterols in tissues and
to lipoproteins in plasma and stays in the body for long periods. The terminal
elimination t½ is 15 days. About 60% of AMB is metabolized in the liver. Excretion
occurs slowly both in urine and bile, but urinary concentration of active drug
is low.
Administration And Dose
Amphotericin B can be administered orally (50–100 mg QID) for
intestinal moniliasis; also topically for vaginitis, otomycosis, etc.: FUNGIZONE OTIC 3% ear
drops.
For systemic mycosis,
it is available as dry powder along with DOC for extemporaneous dispersion
before use: FUNGIZONE INTRAVENOUS, MYCOL 50 mg vial. It is first suspended
in 10 ml water and then diluted to 500 ml with glucose solution (saline tends
to make the suspension coarse). Initially 1 mg test dose is injected i.v. over
20 minutes. If no serious reaction follows, 0.3 mg/kg is infused over 4–8
hours. Daily dose may be gradually increased to 0.7 mg/kg depending on
tolerance of the patient. The total dose of AMB for majority of cases is 3– 4 g
given over 2–3 months.
Intrathecal injection
of 0.5 mg twice weekly has been given in fungal meningitis.
New Amphotericin B Formulations
In an attempt to improve tolerability of i.v. infusion of AMB,
reduce its toxicity and achieve targeted delivery, 3 new lipid formulations of
AMB have been produced.
a) Amphotericin B Lipid Complex (ABLC):
Contains 35% AMB incorporated in
ribbon like pCh. No.s of dimyristoyl phospholipids.
b) Amphotericin B Colloidal
Dispersion (ABCD): Disc shaped pCh. No.s containing
50% each of AMB and cholesteryl sulfate are prepared as aqueous dispersion.
c) Liposomal Amphotericin B (Small
Unilamellar Vesicles; SUV): Consists of 10% AMB
incorporated in uniform sized (60–80 nM) unilamellar
liposomes made up of lecithin and other biodegradable phospholipids.
The special features
of these preparations are:
•
They, except ABCD, produce milder acute
reaction (especially liposomal formulation) on i.v. infusion.
• They can be used in patients not tolerating
infusion of conventional AMB formulation.
•
They have lower nephrotoxicity.
•
They cause minimal anaemia.
• The liposomal preparation delivers AMB
particularly to reticulo-endothelial cells in liver and spleen—especially
valuable for kala azar and in immuno-compromised patients.
However, some
preparations, especially ABLC and ABCD, produce lower AMB levels and their
clinical efficacy relative to conventional formulation appears to be lower.
Though none of the above formulations is more effective in deep mycosis than
conventional AMB, the liposomalAMB produces
equivalent blood levels, has similar clinical efficacy with less acute reaction and renal toxicity. It thus
appears more satisfactory, can be infused at higher rates (3–5 mg/kg/day), but
is many times costlier than conventional AMB. Its specific indications are—as
empirical therapy in febrile neutropenic patients not responding to
antibacterial antibiotics, critically ill deep mycosis cases and in kala azar.
FUNGISOME (liposomal
AMB) 10 mg, 25 mg, 50 mg per vial inj.
Adverse Effects
The toxicity of AMB is high.
a) Acute Reaction: This occurs with each infusion and consists of chills, fever, aches
and pain all over, nausea, vomiting and dyspnoea lasting for 2–5 hour, probably
due to release of cytokines (IL, TNFα). When these are severe—the dose is increased
gradually. Usually the intensity of reaction decreases with continued
medication. Injection of hydrocortisone 0.6 mg/kg with the infusion may reduce
the intensity of reaction.
Thrombophlebitis of
the injected vein can occur.
b) Long-Term Toxicity: Nephrotoxicity is the most important. It occurs fairly uniformly and is doserelated:
manifestations are—azotemia, reduced g.f.r., acidosis, hypokalaemia and
inability to concentrate urine. It reverses slowly and often incompletely after
stoppage of therapy. Anaemia: Most patients develop slowly progressing anaemia
which is due to bone marrow depression. It is largely reversible.
CNS toxicity: occurs
only on intrathecal injection—headache, vomiting, nerve palsies, etc.
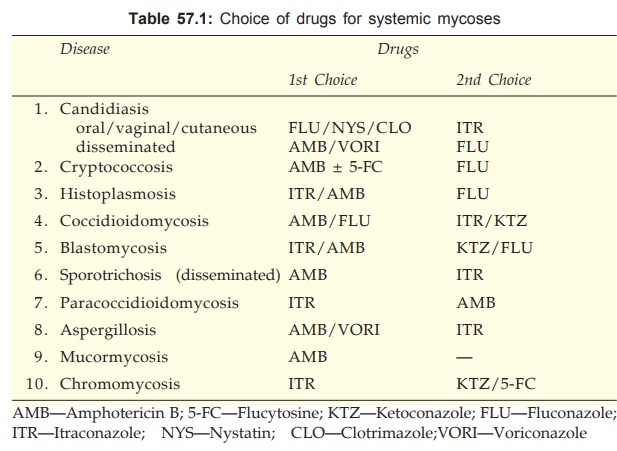
Uses
Amphotericin B can be
applied topically for oral, vaginal and
cutaneous candidiasis and otomycosis.
It is the most
effective drug for various types of systemic mycoses and is the gold standard
of antifungal therapy. However, because of higher toxicity of AMB, the azole
antifungals are now preferred in conditions where their efficacy approaches
that of AMB (see Table 57.1).
Leishmaniasis: AMB is the most
effective drug for resistant cases of
kala azar and mucocutaneous leishmaniasis (see
Ch. No. 60).
Interactions
Flucytosine has supra-additive action with AMB in the case of fungi sensitive
to both (AMB increases the penetration of 5FC into the fungus).
Rifampin and
minocycline, though not antifungal in their own right, potentiate AMB action.
Nystatin
Obtained from S. noursei, it is similar to AMB in
antifungal action and other properties. However, because of higher systemic
toxicity, it is used only locally in superficial candidiasis and is generally
preferred over AMB for these purposes.
MYCOSTATIN 5 lac U
tab, 1 lac U vaginal tab, 1 lac U/ g oint, NYSTIN EYE 1 lac U/g ophthalmic
oint.
Given orally, it is
not absorbed; can be used for monilial diarrhoea (due to superinfection or otherwise),
5 lac U TDS (1 mg = 2000 U). Nausea and bad taste in mouth are the only side
effects.
Nystatin is effective
(but less than azoles) in monilial vaginitis—1 lac U tab inserted twice daily.
For oral thrush, the vaginal tab may be sucked or it may be crushed and
suspended in glycerine for application in mouth. Corticosteroid aerosols (e.g.
beclomethasone) can cause oral candidiasis: nystatin is effective in preventing
as well as treating it.
Similarly, it is used
for corneal, conjunctival and cutaneous candidiasis in the form of an ointment.
No irritation or other side effect is ordinarily seen.
Candidal resistance to
nystatin is not a clinical problem. It is ineffective in dermatophytosis.
Hamycin
It was isolated from S. pimprina and developed by Hindustan
Antibiotics at Pimpri. It is similar to nystatin, but more water soluble. A
fraction of the orally administered dose is absorbed, but cannot be relied upon
for the treatment of systemic mycosis: use is restricted to topical application
for oral thrush, cutaneous candidiasis, monilial and trichomonas vaginitis and
otomycosis by Aspergillus.
HAMYCIN, IMPRIMA 5 lac
U/g oint, 2 lac U/ ml susp for topical use, 4 lac U vaginal ovules.
Natamycin (Pimaricin)
It is similar to
nystatin; has a broader spectrum of
action, and is used only topically. A 5% suspension or 1% ointment is nonirritating
to the eye, and has been used particularly in Fusarium solani keratitis. Both monilial and trichomonas vaginitis
are amenable to natamycin.
NATAMYCIN 2% cream, 25
mg vaginal tab, PIMAFUSIN VAGINAL 100 mg vaginal tab.
