Treatment of Tuberculosis
| Home | | Pharmacology |Chapter: Essential pharmacology : Antitubercular Drugs
The conventional 12–18 month treatment has been replaced by more effective and less toxic 6 month treatment which also yields higher completion rates.
TREATMENT OF TUBERCULOSIS
The therapy of tuberculosis has undergone remarkable change.
The conventional 12–18 month treatment has been replaced by more
effective and less toxic 6 month treatment which also yields higher completion
rates. This has been possible due to better understanding of the biology of
tubercular infection and the differential properties of the antitubercular
drugs.
Biology Of Tubercular Infection
M. tuberculosis is an aerobic organism. In
unfavourable conditions it grows only intermittently or remains dormant for
prolonged periods. Several subpopulations of bacilli, each with a distinctive
metabolic state, could exist in an infected patient, e.g.:
a) Rapidly growing with high
bacillary load as in the wall of a cavitary lesion where oxygen tension is
high and pH is neutral. These bacilli are highly susceptible to H and to a lesser
extent to R, E and S.
b)
Slow growing located
intracellularly (in macrophages) and at inflamed sites
where pH is low. They are particularly vulnerable to Z, while H, R and E are
less active, and S is inactive.
c)
Spurters within caseous
material where oxygen tension is low but pH
is neutral: the bacilli grow intermittently with occasional spurts of active
metabolism. R is most active on this subpopulation.
d)
Dormant some bacilli remain
totally inactive for prolonged periods. No
antitubercular drug is significantly active against them.
However, there is
continuous shifting of bacilli between these subpopulations.
The goals of
antitubercular chemotherapy are:
Kill Dividing
Bacilli: Drugs with early
bactericidal action rapidly reduce bacillary load in the patient and achieve quick
sputum negativity so that the patient is noncontagious to the community:
transmission of TB is interrupted. This also affords quick symptom relief.
Kill Persisting
Bacilli: To effect cure and prevent
relapse. This depends on sterilizing capacity of the drug.
Prevent Emergence Of Resistance: So that the bacilli remain
susceptible to the drugs.
The relative activity
of the first line drugs in achieving these goals differs, e.g. H and R are the most
potent bactericidal drugs active against all populations of TB bacilli, while Z
acts best on intracellular bacilli and those at inflamed sites— has very good
sterilizing activity. On the other hand S is active only against rapidly
multiplying extracellular bacilli. E is bacteriostatic—mainly serves to prevent
resistance and may hasten sputum conversion.
Drug combinations are
selected to maximise the above actions together with considerations of cost,
convenience and feasibility. The general principles of antitubercular
chemotherapy are:
· Use of any single drug in tuberculosis results
in the emergence of resistant organisms and relapse in almost 3/4th patients. A
combination of two or more drugs must be used. The rationale is: the incidence
of resistant bacilli to most drugs ranges from 10–8 to 10–6. Because an average
patient of pulmonary tuberculosis harbours 108 to 1010 bacilli, the number of
organisms that will not respond to a single drug is high and cannot be dealt by
the host defence. During protracted treatment, these bacilli multiply and
become dominant in 3–4 months. Because insensitivity to one drug is independent
of that to another, i.e. incidence of H resistance among bacilli resistant to R
will be 10–6 and vice versa; only few
bacilli will be resistant to both; these can be handled by host defence. By the
same rationality, massive infection (>1010 organisms) has to be treated by
at least 3 drugs; and a single drug is sufficient for prophylaxis, because the number
of bacilli is small.
· Isoniazid and R are
the most efficacious drugs; their combination is definitely synergistic—
duration of therapy is shortened from > 12 months to 9 months. Addition of Z
for the initial 2 months further reduces duration of treatment to 6 months.
· A single daily dose of
all first line antitubercular drugs is preferred. The ‘directly observed
treatment short course’ (DOTS) was recommended by the WHO in 1995.
· Response is fast in
the first few weeks as the fast dividing bacilli are eliminated rapidly.
Symptomatic relief is evident within 2–4 weeks. The rate of bacteriological,
radiological and clinical improvement declines subsequently as the slow
multiplying organisms respond gradually. Bacteriological cure takes much
longer. The adequacy of any regimen is decided by observing sputum conversion
rates and 2–5 year relapse rates after completion of treatment.
Conventional Regimens
These consist of H + Tzn or E with or without S (for
initial 2 months) and require 12–18 months therapy. Failure rates are high,
compliance is poor—therefore not recommended now.
SHORT COURSE CHEMOTHERAPY (SCC)
These are regimens of
6–9 month duration which have been found highly efficacious. After several
years of experience a WHO expert group has framed clearcut treatment guidelines*
(1997) for different categories of TB patients. The dose of first line antiTB
drugs has been standardized on body weight basis and is applicable to both
adults and children (Table 55.1).
All regimens have an initial intensive phase, lasting for 2–3
months aimed to rapidly kill the TB bacilli, bring about sputum conversion and
afford symptomatic relief. This is followed by a continuation phase lasting for 4–6 months during which the remaining bacilli are
eliminated so that relapse does not occur. Treatment of TB is categorized by:
· Site of disease (pulmonary or extrapulmonary)
and its severity: the bacillary load and acute threat to life or permanent
handicap are taken into consideration.
· Sputum smear positivity/negativity: positive
cases are infectious and have higher mortality.
· History of previous treatment: risk of drug
resistance is more in irregularly treated patients.
Rationale Patients of smear-positive
pulmonary TB harbour and
disseminate large number of![]() bacilli. Initial treatment with 4 drugs
reduces risk of selecting resistant bacilli as well as covers patients with
primary resistance. When few bacilli are left only 2 drugs in the continuation
phase are enough to effect cure. Smear-negative pulmonary TB and extrapulmonary
TB patients harbour fewer bacilli in their lesions—risk of selecting resistant
bacilli is less; regimens containing only 3 drugs in the initial phase and 2 in
the continuation phase are of proven efficacy. Accordingly, previously
treated/failure/default/ relapse cases are treated with a longer intensive
phase—5 drugs for 2 months and 4 drugs for 1 month followed by 3 drugs in the
continuation phase of 5 months duration (instead of usual 4 months).
bacilli. Initial treatment with 4 drugs
reduces risk of selecting resistant bacilli as well as covers patients with
primary resistance. When few bacilli are left only 2 drugs in the continuation
phase are enough to effect cure. Smear-negative pulmonary TB and extrapulmonary
TB patients harbour fewer bacilli in their lesions—risk of selecting resistant
bacilli is less; regimens containing only 3 drugs in the initial phase and 2 in
the continuation phase are of proven efficacy. Accordingly, previously
treated/failure/default/ relapse cases are treated with a longer intensive
phase—5 drugs for 2 months and 4 drugs for 1 month followed by 3 drugs in the
continuation phase of 5 months duration (instead of usual 4 months).
The categorywise treatment regimens are summarized in Tables 55.2 and 55.3.
Category I
This category
includes:
·
New (untreated) smear-positive pulmonary TB.
·
New smear-negative pulmonary TB with extensive
parenchymal involvement.
·
New cases of severe forms of extrapulmonary
TB, viz. meningitis, miliary,
pericarditis, peritonitis, bilateral or extensive pleural effusion, spinal,
intestinal, genitourinary TB.
Initial Phase
Four drugs HRZ + E or
S are given daily or thrice weekly
for 2 months. The revised national tuberculosis control programme (RNTCP) has
been launched in India in 1997, which is implementing DOTS*. Out of the WHO
recommended regimens, the RNTCP has decided to follow thrice weekly regimen,
since it is equally effective, saves drugs and effort, and is more practical.
The RNTCP regimen is presented in Table 55.3. The RNTCP recommends that if the
patient is still sputumpositive at 2 months, the intensive phase should be
extended by another month; then continuation phase is started regardless of
sputum status at 3 months.
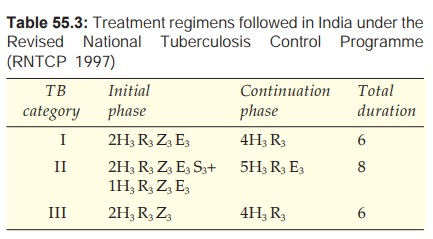
· Each antiTB drug has a standard
abbreviation (H, R, Z, E, S).
· The numeral before a phase is the
duration of that phase in months.
· The numeral in subscript (e.g. H3
R3) is number of doses of that drug per week. If there is no
subscript numeral, then the drug is given daily.
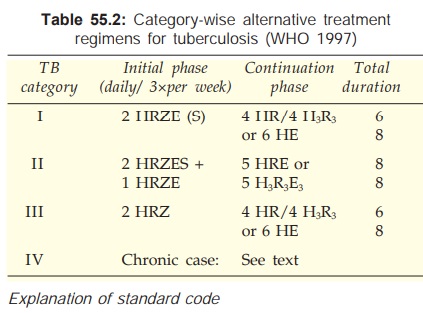
Continuation
Phase
Two drugs HR for 4 months or HE for 6 months are given. When both H and
R are used, thrice weekly regimen is permissible. Under the RNTCP, thrice
weekly treatment with H and R is given for 4 months. This phase is extended to
6–7 months (total duration 8–9 months) for TB meningitis, miliary and spinal
disease. In areas where DOTS has not been implemented, use of Tzn in place of E
in the continuation phase is permitted except in HIV positive cases.
Category
II
These are smear-positive
failure, relapse and
interrupted treatment cases:
Treatment failure: Patient who remains or again becomes smear-positive 5 months or
later after commencing treatment. Also one who was smear-negative at start of
therapy and becomes smear-positive after the 2nd month.
Relapse:
A patient declared cured from any form of TB in the past after receiving one full course of chemotherapy
and now has become sputum positive.
Treatment After Interruption (Default): A patient who interrupts treatment for 2 months or more and returns with sputum-positive
or clinically active TB.
These patients may have resistant bacilli and are at greater
risk of developing MDRTB.
Initial phase
All 5 first line drugs
are given for 2 months followed by 4
drugs (HRZE) for another month. Continuation phase is started if sputum is negative,
but 4 drug treatment is continued for another month if sputum is positive at 3
months.
Continuation phase
Three drugs (HRE) are given for 5 months either
daily or thrice weekly (only thrice weekly under the RNTCP).
Category III
These are new cases of smear-negative pulmonary TB with limited
parenchymal involvement or less severe forms of extrapulmonary TB, viz, lymph node TB, unilateral pleural
effusion, bone (excluding spine), peripheral joint or skin TB.
Initial Phase
Three drugs (HRE) given for 2 months are enough because
the bacillary load is smaller.
Continuation Phase
This is similar to
category I, i.e. 4 month
daily/thrice weekly HR or 6 months daily HE (Tzn) therapy. Under the RNTCP only
thrice weekly HR regimen is followed.
Category IV
These are chronic cases who have remained or have
become smear-positive after completing fully supervised retreatment (Category
II) regimen. These are most likely MDR cases.
Multi-Drug-Resistant (MDR) TB is defined as resistance to both H and R and may be any number
of other anti-TB drugs. MDRTB has a more rapid course (some die in 4–16 weeks).
Treatment of these cases is difficult, because one or more second line drugs
are to be given for 12–24 months. The second line drugs are less efficacious,
less convenient, more toxic and more expensive.
The choice of drugs
depends on the drugs used in the earlier regimen, dosage and regularity with
which they were taken, presence of associated disease like AIDS/diabetes/leukaemia/silicosis,
and whether sensitivity of the pathogen to various drugs is known (by in vitro testing) or unknown. If
sensitivity of the TB bacilli is known, the drug/ drugs to which they are
resistant is/are excluded and other first line drugs are prescribed along with
1–3 second line drugs. A total of 5–6 drugs are given. One of the FQs is
generally included. In case streptomycin is not being given, one out of
kanamycin/amikacin/capreomycin should be added, because they are
tuberculocidal.
a)
For H resistance—RZE given for 12 months is
recommended.
b)
For H + R resistance—ZE + S/Kmc/Am/Cpr +
Cipro/ofl ± Etm could be used.
The actual regimen is
devised according to the features of the individual patient.
Extensively Drug Resistant (XDR) TB
Recently, the WHO and CDC (USA) have identified TB cases
that are ‘extensively drug resistant’. This term has been applied to bacilli
that are resistant to at least 4 most effective cidal drugs, i.e. cases
resistant to H, R, a FQ, one of Kmc/Am/Cpr with or without any number of other
drugs. The global survey for the period 20022004 has found 20% TB isolates to
be MDR, out of which 2% were XDR. The XDRTB is virtually untreatable; mortality
is high, particularly among HIV positive patients.
Tuberculosis In Pregnant Women
The WHO and British Thoracic
Society consider H, R and Z to be safe to the foetus and recommend the standard
6 month (2HRZ + 4HR) regimen for pregnant women with TB. E can be added during
late but not early pregnancy. S is contraindicated. However, Z is not
recommended in the USA (due to lack of adequate teratogenicity data). In India,
it is advised to avoid Z, and to treat pregnant TB patients with 2 HRE + 7HR
(total 9 months). Treatment of TB should not be withheld or delayed because of
pregnancy.
Treatment Of Breastfeeding Women
All antiTB drugs are
compatible with breastfeeding; full course should be given to the mother, but
the baby should be watched (See
Appendix3). The infant should receive BCG vaccination and isoniazid
prophylaxis.
Management Of Patients With
Adverse Drug Reactions To Antitubercular Drugs
Minor side effects are to be
managed symptomatically without altering medication; e.g. Z induced arthralgia
can be treated by analgesicNSAIDs; peripheral neuritis due to H can be counteracted
by pyridoxine. With more severe reactions, the offending drug should be stopped;
e.g. E should be promptly discontinued at the first indication of optic
neuritis. If possible H and R should be continued, or should be reintroduced
after the reaction has subsided by challenging with small doses. However, R
should never be reintroduced in case of severe reaction such as haemolysis,
thrombocytopenia or renal failure.
Hepatotoxicity is the most common problem with antitubercular
drugs. Any one or more of H, R and Z could be causative and the reaction occurs
more frequently when combination of these drugs is used. In case hepatitis
develops, all these drugs should be stopped and S + E may be started or
continued. A fluoroquinolone may be added. When the reaction clears, the above
drugs are started one by one to identify the culprit, which should never be
used again, while the others found safe should be continued. It is best to
avoid Z in patients who once developed hepatitis.
Chemoprophylaxis
The purpose is to prevent progression of latent
tubercular infection to active disease. This is indicated only in :
a)
Contacts of open cases who show recent Mantoux
conversion.
b)
Children with positive Mantoux and a TB
patient in the family.
c)
Neonate of tubercular mother.
d)
Patients of leukaemia, diabetes, silicosis, or
those who are HIV positive but are not anergic, or are on corticosteroid
therapy who show a positive Mantoux.
e) Patients with old inactive disease who are
assessed to have received inadequate therapy.
The standard drug for
chemoprophylaxis of TB is H 300 mg (10 mg/kg in children) daily for 6–12
months. This is as effective in HIV patients as in those with normal immune
function. However, because of spread of INH resistance, a combination of H (5
mg/kg) and R (10 mg/kg) daily given for 6 months is preferred in some areas.
The CDC (USA) recommends 4 months R prophylaxis in case H cannot be used. An
alternative regimen is daily R + Z for 2 months, but this carrys risk of severe
liver damage, and needs close monitoring. Therefore, it is reserved for
contacts of resistant TB cases. Another regimen for subjects exposed to MDRTB
is E + Z with or without a FQ.
Role of corticosteroids
Corticosteroids should not be ordinarily used in tubercular patients.
However, they may be used under adequate chemotherapeutic cover:
a) In seriously ill patients (miliary or severe
pulmonary TB) to buy time for drugs to act.
b) When hypersensitivity reactions occur to
antitubercular drugs.
c) In meningeal or renal TB or pleural effusion—
to reduce exudation and prevent its organisation, strictures, etc.
d) In AIDS patients with severe manifestations of
tuberculosis.
Corticosteroids are contraindicated
in intestinal tuberculosis—silent perforation can occur. Corticosteroids, if
given, should be gradually withdrawn when the general condition of the patient
improves.
Tuberculosis In AIDS Patients
The association of HIV and TB infection is a serious problem.
HIV positive cases have more severe and more infectious TB. HIV infection is
the strongest risk factor for making latent TB overt. Moreover, adverse
reactions to antiTB drugs are more common in HIV patients.
On the other hand, institution of ‘highly active antiretroviral
therapy’ (HAART) and improvement in CD4 cell count markedly reduces the incidence
of TB among HIVAIDS patients. When CD4 count is <150 cells/μL, extrapulmonary and
dual TB is more commonly encountered.
In case of M. tuberculosis infection, drugs used
are the same as in non-HIV cases, but the duration is longer and at least 4
drugs are used. Initial therapy with 2 month HRZE is started immediately on the
diagnosis of TB, and is followed by a continuation phase of HR for 7 months
(total 9 months). Alternatively, 3 drugs (HRE) are given for 4 months in the
continuation phase. Pyridoxine 25–50 mg/day is routinely given along with H to
counteract its neurological side effects, which are more likely in AIDS patients.
Consideration also has
to be given to possible drug interactions between anti-TB and antiretroviral
(ARV) drugs. Rifampin, a potent inducer of CYP isoenzymes, markedly enhances
the metabolism of protease inhibitors (PIs, viz.
indinavir, nelfinavir, ritonavir) and of NNRTIs, viz. nevirapine, efavirenz, to a lesser extent, making them ineffective. In patients receiving
these drugs, rifabutin (a less potent enzyme inducer) given for 9–12 months may
be substituted for rifampin. The metabolism of nucleoside reverse transcriptase
inhibitors (NRTIs, zidovudine, etc.) is not induced by rifampin—no dose
adjustment is needed. An alternative regimen of 3 NRTIs (zidovudine +lamivudine
+ abacavir) has been advocated for patients who are to be treated by rifampin.
MDRTB in HIVAIDS
patients should be treated for a total of 18–24 months or for 12 months after
sputum smear negativity.
Mycobacterium avium complex (MAC) infection is common in HIVAIDS
patients, particularly when the CD4 count drops to < 100 cells/μL.
Clarithromycin/azithromycin are the most active drugs against MAC. A favoured
regimen consists of an intensive phase of at least 4 drugs—
clarithromycin/azithromycin + ethambutol + rifabutin + one FQ/clofazimine/ethionamide
given for 2–6 months (duration is response based), followed by 2 drug
maintenance phase with clarithromycin/azithromycin + ethambutol/one
FQ/rifabutin for at least 12 months or even lifelong. However, any additional
benefit of the initial 4 drug intensive phase is unproven. Clarithromycin
inhibits the metabolism of rifabutin.
Prophylaxis of MAC in
AIDS patients by clarithromycin/azithromycin (or rifabutin if these drugs
cannot be given) is advocated when the CD4 count falls below 100 cells/μL. After institution
of HAART, this is continued till near complete suppression of viral replication
is achieved and CD4 count rises above 100 cells/ μL. Otherwise,
prophylaxis is continued lifelong.
Related Topics
