Reporting Volumes
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Spontaneous Reporting - UK
Since the launch of the Yellow Card Scheme in 1964, over 500,000 reports have been received by the MHRA and the CSM from health professionals, either directly through the Scheme or indirectly via phar-maceutical companies.
REPORTING VOLUMES
Since
the launch of the Yellow Card Scheme in 1964, over 500,000 reports have been
received by the MHRA and the CSM from health professionals, either directly
through the Scheme or indirectly via phar-maceutical companies (Figure 15.1).
The Scheme is voluntary for health professionals but pharmaceutical companies
have legal obligations to report ADRs to the MHRA (Waller, Coulson and Wood,
1996), and in 2003 and 2004 the latter accounted for approx-imately 30% of all
ADR reports received. It can be seen that the annual number of reports has
risen significantly since the introduction of the Scheme, with notable
increases in reporting in the mid-1970s and again in 1986. The first of these
increases coin-cided with the withdrawal of practolol following its association
with oculomucocutaneous syndrome, the introduction of the CSM drug safety
bulletin Current Problems in Pharmacovigilance, and the inclusion of a yellow page in prescription pads
used by GPs, reminding them to report ADRs. The second increase is thought to
have resulted from the increased avail-ability of Yellow Cards to doctors,
following their inclusion in the British National Formulary (BNF), which is
supplied to all doctors, and in prescription pads (Rawlins, 1988a).
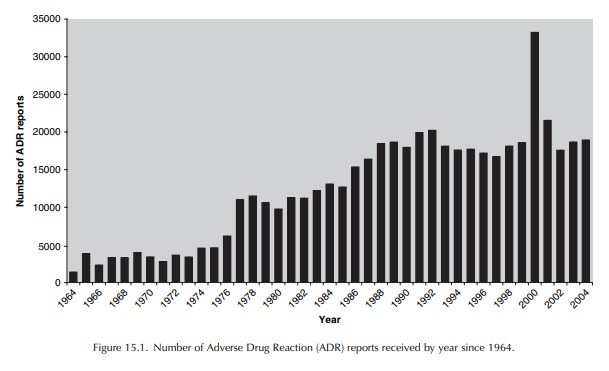
There
was a significant change in the early 1990s when the annual number of Yellow
Cards declined from a peak of just over 20 000 to an annual aver-age of around
17 000 in the mid- to late 1990s. A number of factors may be responsible for
contribut-ing to this decline; for instance, the number of Yellow Cards
submitted on forms included in GPs’ prescription pads has fallen dramatically
in the past 10 years (these ‘FP10’ forms comprised 10% of all UK reports
received in 1991, compared with 0.1% in 2001), suggesting a move from
handwrit-ten prescriptions to increasing use of computerised practice systems.
Additional factors may include the increasing demands on doctors time and
concerns over confidentiality, as evidenced by surveys of factors affecting
reporting as described above.
GP
focus groups have been used to examine under-standing of, and attitudes to,
ADRs and report-ing via the Yellow Card Scheme. The key find-ings were broadly
in line with published surveys of attitudes to ADR reporting, namely that GPs
were too busy to report, and that they were uncer-tain about how to distinguish
adverse reactions from adverse events. Additionally, there was some concern
about confidentiality issues associated with supply-ing patient details, and
uncertainty about where ADR reports were sent and how the information would be
used.
In
2000, there was a dramatic rise in the number of Yellow Cards, with over 33 000
reports received during this 12-month period. This can largely be accounted for
by the reporting of a large number of suspected adverse reactions to meningitis
C vaccines, administered to children under the age of 18 in a nationwide
immunisation campaign. Nurse reporting was permitted during the campaign and an
estimated 18.5 million doses of vaccine were distributed in just over a year.
Even when reports for this vaccine are excluded, there was a 16% rise in the
number of Yellow Cards received in 2000 compared with that of 1999.
Following
completion of the meningitis C immunisation campaign, the number of Yellow
Cards returned to previous levels. In 2003 and 2004 the number of reports have
steadily increased and this coincides with the formal introduction of nurse,
midwife and health visitor reporting in October 2002 and the introduction of
electronic reporting during the same time period. It remains to be seen if this
level of healthcare professional reporting will be maintained in future years,
especially with the introduction of patient reporting.
Related Topics
