Risks with medicines use
| Home | | Hospital pharmacy |Chapter: Hospital pharmacy : Risks with medicines
In addition to the potential for harm from adverse drug reactions to medicines when used correctly, patients may be harmed as a result of incorrect medicines use.
Risks with medicines use
In addition to the
potential for harm from adverse drug reactions to medicines when used
correctly, patients may be harmed as a result of incorrect medicines use.
Awareness of the
risks associated with healthcare has risen over the past decade following the
publication in 2000 of To Err is Human in the USA by the Institute of Medicine.
This milestone document acknowledged that healthcare is not as safe as it
should be and pointed to medical errors, includ-ing medication errors, being a
leading cause of death and injury. One of the events which triggered this
publication was the death of a Boston Globe health reporter from an accidental
overdose of chemotherapy.
In England, in 2000
the Department of Health published its own account of patient safety in the
NHS. An Organisation with a Memory was written by an expert panel, chaired by
the chief medical officer.5 It recognised that serious adverse
events had been allowed to recur within the NHS due to a lack of capacity to
learn from infrequent but devastating events.
A specific example
used to illustrate the problem was the fatal conse-quence of inadvertent spinal
injection of vinca alkaloids intended for intra-venous administration in
chemotherapy regimens for the treatment of some haematological cancers. Between
1985 and publication of the report, 12 cases of maladministration of
intravenous vinca alkaloids were known to have occurred, of which 10 were known
to have resulted in either death or paralysis of the patient. The outcomes in
the other two were unknown. Despite the known toxicity of vinca alkaloids, and
warning labels on products, these preventable errors were being repeated with
devastating consequences for patients, their families and the staff involved in
their care.
An Organisation with
a Memory recommended the establishment of an independent scheme for mandatory
reporting of adverse healthcare events and near-misses to which staff could
report confidentially. It recommended a single system for analysing and
disseminating lessons learnt from these adverse events, making recommendations
to improve patient safety and encouraging further reporting. The implementation
of these recommenda-tions was described in Building a Safer NHS for Patients.
Published in 2001, Building a Safer NHS for Patients: Implementing an
Organisation with a Memory further recognised the complexity of healthcare and
the risks associated with it. Patient safety was identified as a worldwide
problem, and the need to improve safety in healthcare by strengthening systems
through capturing data on error and learning from the analysis of incidents was
described.
More specifically,
the document established the National Patient Safety Agency (NPSA) and listed
four key areas that should be the focus for change, two of which specifically
referred to the use of medicines. The two targets for medication safety were
to:
·
reduce to zero the number of patients dying or being
paralysed by maladministered spinal injections by the end of 2001
·
reduce by 40% the number of serious errors in the use of
prescribed drugs by 2005.
The way in which the
NHS should work towards achieving these two targets was described in two
subsequent documents, The Prevention of Intrathecal Errors and Building a Safer
NHS for Patients: Improving Medication Safety.
The report into the
prevention of intrathecal errors described two main strategies for error
prevention: (1) human factors, encompassing training and education, ward and
pharmacy procedures and policies; and (2) design changes. The concept of design
or engineering safety was a new concept in risk management in healthcare at the
time. The report highlighted the inherent risk of misconnection due to the
universal use of a Luer connector as a root cause of the error, compounded in
some situations by human actions. The themes of root cause analysis (RCA) and
design change have since become embedded into the processes for investigating
serious adverse events in the NHS.
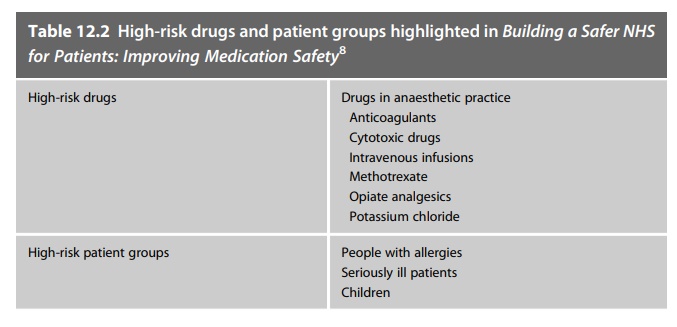
Building a Safer NHS
for Patients: Improving Medication Safety addressed the target of reducing the
number of serious errors in the use of prescribed medicines. It described the
published literature on medication safety and highlighted the processes,
medicines and situations known to be associated with harm. The document
recommended actions to be taken by NHS organisations to recognise and reduce
these risks, although at the time these had not been accurately quantified. The
publication drew attention to drugs known to be harmful and patient groups who
were known to be at risk of harm from medicines: Table 12.2 lists these.
Related Topics
