Screening Methods for Antifertility Agents
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : Biological Screening of Herbal Drugs
Antifertility agents are substances which prevent reproduction by interfering with various normal reproductive mechanisms in both males and females. An ideal contraceptive agent is one which possess 100% efficacy, reversibility of action, which is free from side effects and is easy to use.
SCREENING METHODS FOR ANTIFERTILITY AGENTS
Antifertility agents are substances which prevent
reproduction by interfering with various normal reproductive mechanisms in both
males and females. An ideal contraceptive agent is one which possess 100%
efficacy, reversibility of action, which is free from side effects and is easy
to use.
Ancient literature has mentioned the use of a number of
plants/preparations for regulation of fertility in the form of emmenagogues,
ecbolics, abortifacients, and local contraceptives. For centuries, virtually
every indigenous culture has been using plants and/or their various parts in
one or the other form to restrict its population. Women have used herbs since
time immemorial to control their fertility. The information was passed on from
mother to daughter; midwives and wise women all possessed this knowledge, but
most of these plant’s activities and their mechanism of action were not
scientifically studied.
There are approximately 2,50,000 species growing on earth.
It stands to reason that not all of them can be used to regulate fertility;
therefore some criteria have to be laid down for selecting plants to evaluate
their antifertility potential. Three options are available:
1. Investigation of plants that have
folkloric/traditional reputation as contraceptives;
2. Evaluation of plants that are known
to contain con-stituents which theoretically affect the female cycle and thus
produce antifertility effects, for example, oestrogenic sterols, isoflavones,
and coumestans or those, which have a potential to contract the uterus; and
3. Random collection of plants for mass
screening.
During the last six decades, sporadic attempts have been
made by Indian investigators to evaluate antifertility plants. But there is
variation in the reports given by various inves-tigators on the same plant part
(from inactivity to 100% activity). This appears to be due to inadequate
attention given to proper botanical identification, authentication, and testing
procedure. In spite of the detailed description of plants found in ancient
ayurvedic and unani literature, documented experimental or clinical data on
them are lacking. Furthermore the efficacies of these plants have not yet been
confirmed through repeated investigations.
Screening Methods for Antifertility Activity in
Females
Antifertility action of drugs acting in females may be due
to:
1. Inhibition of ovulation
2. Prevention of fertilization
3. Interference with transport of ova
from oviduct to endometrium of the uterus
4. Implantation of fertilized ovum
5. Distraction of early implanted
embryo
Screening Methods for Antiovulatory Activity
Cupric
acetate-induced ovulation in rabbits
Rabbits are reflex ovulators. They ovulate within a few
hours after mating or after mechanical stimulation of vagina or sometimes even
the mere presence of a male or administration of certain chemicals like cupric
acetate.
In this screening method, cupric acetate is used for the
induction of ovulation. The rabbit ovulates within a few hours after an i.v.
injection of cupric acetate (0.3 mg/ kg using 1% cupric acetate in 0.9%
saline). Injection of antiovulatory drugs, 24 h before the induction procedure
prevents ovulation.
Sexually mature female albino rabbits, weighing 3–4 kg, are
used for the study. Animals are kept in isolation for at least 21 days to
ensure that they are not pregnant and to prevent the induction of ovulation by
mating. They are then treated with the test drug, and 24 h later an i.v.
injection of cupric acetate is given. The rabbits are sacri-ficed and the
ovaries are examined 18–24 h later. The total number of ovulation points on
both the ovaries is recorded for each animal. Then the ovaries and uterus are excised
and preserved in 10% buffered formalin and subjected to histopathological
evaluation.
HCG-induced
ovulation in rats
Immature female albino rats do not ovulate spontaneously and
do not show cyclic changes of the vaginal epithe-lium. Priming with human
chorionic gonadotropin (HCG) induces follicular maturation, followed by
spontaneous ovulation two days later. Injection of an antiovulatory drug before
the induction procedure will prevent ovulation. This principle is used for
screening potential antiovula-tory agents.
Immature female albino rats (24–26 days old) are used for
the experiment. The animals are treated with various test drugs in different
dose levels. After the administration of the test drug, exogenous HCG is given
to induce ovula-tion. After two days, the animals are sacrificed. Their ovaries
are preserved in a 10% buffered formalin and subjected to histopathological
evaluation. The results are compared with the control group.
Screening Methods for Oestrogenic Compounds: In Vivo
Methods
A primary therapeutic use of oestrogen (both in vivo and in
vitro) is in contraception. The
rationale for these prepara-tions is that excess exogenous oestrogen inhibits
FSH and LH and thus prevents ovulation.
Assay
for water uptake
The principle of the assay is based on the observation that
the uterus responds to oestrogens by increased uptake and retention of water. A
peak in the uptake is observed six hours after administration.
Ovariectomized adult animals may be used for this
experiment. It is simpler to use immature 18-day-old mice or 22-day-old rats
obtained two days before the beginning of the experiment. The animals are
randomly grouped. The control group is given 0.1 ml of cottonseed oil (vehicle
for estradiol) subcutaneously. The oestrogen control group is given doses
ranging from 0.01 to 0.1 μg to establish a dose-response
curve. In the initial test, the test compound is given to groups at a high and
low dose. In subsequent tests, it is given over a range of doses to provide the
dose-response curve. All doses are given in 0.1 ml of cottonseed oil.
Five hours after treatment, the animals are killed by
cer-vical fracture and the uteri are quickly excised. The opera-tion is begun
by a longitudinal slit through the skin of the abdomen and through the body
wall. The uterus is picked up with the forceps and severed from the vagina. The
uterine horns are separated from the connective tissues and are then cut at
their constriction point near the ovary. The uteri are kept moist by placing
them on dump (not wet) filter paper and by covering them with damp filter
paper. They are then rapidly weighed in a sensitive balance. The uteri are
dried in an oven at 60°C, for 24 h and are reweighed. The percentage increase
in water over control can be calculated and compared with the values of other
groups.
Procedure for ovariectomy: The animals are anaesthetized with ether. A single transverse
incision is made in the skin of the back. This incision can be shifted readily
from one side to the other, so as to lie over each ovary in turn. A small
puncture is then made over the site of the ovary, which can be seen through the
abdominal wall, embed-ded in a pad of fat. The top of a pair of fine forceps is
introduced, and the fat around the ovary is grasped, care being taken not to
rupture the capsule around the ovary itself. The tip of the uterine horn is
then crushed with a pair of artery forceps and the ovary together with the
fal-lopian tube removed with a single cut using a pair of fine scissors.
Usually no bleeding is observed. The muscular wound is closed by absorbable
sutures, and the outer skin wound is closed by nylon suture.
Four-day
uterine weight assay
This assay is based on the observation that oestrogens cause
an increase in protein synthesis and thus bring about an increase in uterine
weight. A peak is observed after about 40 h.
Immature or Ovariectomized albino mice or rats can be given
the test drug intramuscularly in cottonseed oil for three consecutive days. On
the fourth day, animals are killed by cervical fracture, the uteri rapidly
excised, and the uterine contents gently squeezed out (results are unreli-able
if the uterine contents are not removed). The uteri are weighed immediately in
the wet state. They are then dehydrated in an oven at 100°C for 24 h and
reweighed to obtain the dry weight increase. The log dose is plotted against
the wet weight to produce a sigoid curve, and the ED50 can be
determined for comparison of the test com-pound with estradiol.
Vaginal
opening
This assay is based on the principle that vaginal opening
occurs in immature female albino mice and rats when treated with oestrogenic
compounds. Complete vaginal opening is considered a sign of oestrogenic
activity.
Immature female animals (18-day-old mice, 21-day-old rats)
are used for the study. The test and standard drugs are administered to the
animals intramuscularly in cot-tonseed oil. The vaginal opening is observed to
determine oestrogenic activity.
Vaginal
cornification
This assay is based on the fact that rats and mice exhibit a
cyclical ovulation with associated changes in the secretion of hormones. This
leads to changes in the vaginal epithelial cells. The estrus cycle is
classified into the proestrus, estrus, metestrus, and diestrus stage. Drugs
with oestrogenic activity change the animals from whatever stage they were into
the estrus stage.
Adult female albino rats having a regular estrus cycle are
used for the study. Animals are treated with various test and standard drugs.
Change in the vagina can be observed by taking vaginal smears and examining
these for cornified cells, leucocytes, and epithelial cells in the normal
animals and treated animals twice daily over a period of four days. Any drug
which changes the animals into the estrus stage skipping other stages is
considered to have oestrogenic activity.
The
experimental procedure for taking vaginal smears
Holding the animal on the ventral side up, a drop of normal
saline is inserted into the vagina with a Pasteur pipette. Care must be taken
to avoid damage or injury to the vagina so as to prevent pseudopregnancy. The
drop of normal saline should be aspirated and replaced several times. It is
then transferred to a microscope slide and allowed to dry. The smears are fixed
by placing the slide in absolute alcohol for 5 sec, allowing it to dry, and
staining it with a 5% aqueous methylene blue solution for 10 min. The excess
stain is washed off with tap water, and the slide is dried and observed using a
low power microscope.
Chick
oviduct method
The weight of the oviduct of young chicken increases
depending on the dose of natural and synthetic oestrogen. This principle is
used for the screening of oestrogenic compounds.
Seven-day-old pullet chicks are injected subcutaneously
twice daily with solutions of the test compound in various doses for six days.
Doses (0.02–0.5 μg) of 17β-estradiol per animal serve as standard. Six to ten chicks
are used for each dosage group. On the day after the last injection, the
animals are sacrificed and the weight of the body and oviduct is determined.
Screening Methods for Oestrogenic Compounds: In Vitro
Methods
Potency
assay
This assay determines the affinity of the test compound for
oestrogen receptor sites in the uterus.
The uptake of titrated estradiol by immature uteri must be
established. The inhibition of this uptake by pretreatment with a test compound
will then indicate the oestrogenic potency of the compound.
Four immature female mice (20 days old) are killed. The
uteri are quickly excised and are placed in a Krebs–Ringer phosphate buffer.
Pieces of diaphragm are taken from each animal to serve as control tissue for
nonspecific uptake of estradiol. The uteri are divided at the cervix into two
horns; this helps as one horn can be used as the control and the other for
testing the compound. The tissues are placed in vials containing 5.0 ml of
Krebs–Ringer phosphate buffer, incubated, and shaken at 37°C with 95% oxygen;
5% carbon dioxide is bubbled through. The radiochemical purify of the 3H-estradiol
can be checked chromatographi-cally. Buffer solution of radioactive estradiol
is made up so that each 5 ml of buffer contains 0.0016 μg of radioactive estradiol (0.25 μci). A stock solution can be made and kept refrigerated for
up to 6 weeks.
The excised tissues are treated as follows:
Control: Four pieces of diaphragm are
incubated and shaken with 5 ml of
buffer solution for 15 min at 37°C and are then shaken for 1 h with 5 ml of
buffer containing the radioactive estradiol and 2% w/v bovine albumin.
Experimental: Four uterine horns are incubated and
are shaken in 5 ml of buffer at 37°C
for 15 min. They are then incubated and shaken with 5 ml of buffer containing
2% of albumin and radioactive estradiol at 37°C for 1 h. Both control and
experimental tissue are removed and washed with buffer at 37°C for 5 min, kept
in damped filter paper, and weighed. The tissues are then prepared for
counting. Samples of 100 μl of the incubation solution are
also taken for counting.
Treatment of tissues for counting: The tissues are dried to determine constant weight and the dry
weight recorded. Each piece of tissue is placed in a glass counting vial and
incubated at 60°C in a shaking water bath with 0.5 ml of hyamine hydrochloride
l0x until the tissue has completely dissolved. If the solution is discoloured,
50 μl of 20% hydrogen peroxide may be added. A total of 50 μl of con-centrated HCl and 15 ml of phosphor solution are
added to each vial. The vials are allowed to equilibrate in the packed liquid
scintillation counter, and counts are taken. Counting efficiency is determined
by the addition of an internal standard. The results are expressed as
disintegra-tions per minutes per unit of wet weight (dpm/mg). Test compounds
can be incubated with the labelled oestrogen. This helps in assaying their
effectiveness in competing for the receptors in the uterus.
Oestrogen
receptor-binding assay
Oestrogen receptor-binding assay uses the principle of
competitive binding of labelled and unlabelled oestrogen on the oestrogenic
receptors. Oestrogenic compounds dis-place the labelled oestrogen in a
concentration-dependent manner from the oestrogen receptor.
Cytosol preparation: Uteri from 18-day-old female albino mice are removed and homogenized at 0°C
in 1:50 (w/v) of Tris-sucrose buffer in a conical homogenizer. Human
endometrium from menopausal women frozen within 2 h of hysterectomy and stored
in liquid nitrogen can also used. The frozen endometrium is pulverised and
homog-enized in l:5 (w/v) of Tris-sucrose buffer. Homogenates are centrifuged
for 1 h at 105,000 r.p.m.
Screening Methods for Antioestrogens:
In
Vivo Methods
Antagonism
of physiological effects of oestrogens
Antioestrogenic compounds inhibit some or all of the
physiological effect of oestrogen, such as water uptake of uterus, uterotrophy,
and vaginal cornification. This principle is used for the screening of
antioestrogenic activity.
The assay techniques used for antioestrogens are
modi-fications of the oestrogenic assays. The dose of oestrogen used is that
which is required to produce 50% of the maximum possible response. The test
compound can be injected simultaneously or at varying times before or after the
oestrogen. The procedure for assays of water uptake, uterotrophy, and vaginal
cornification are followed as described earlier except that the test compounds
are given with the oestrogen.
Screening Methods for Antioestrogens:
In
Vitro methods
Aromatase
inhibition
This assay is based on the principle that some compounds
which inhibit aromatase (oestrogen synthase) can produce antioestrogenic
activity. Antioestrogenic activity of com-pounds can be evaluated indirectly by
evaluating aromatase-inhibiting ability.
Ovarian tissue from adult golden hamsters is used. The
estrus cycle is monitored for at least three consecutive four-day estrus cycles
before the experiment. The experiments for evaluating inhibitor effects are
performed with ovaries obtained from animals sacrificed on day 4 (proestrus) of
the cycle. The ovaries are excised free from adhering fat tissue and quartered.
The quarters are transferred into plastic incubation flasks with 2 ml of
Krebs–Ringer bicarbonate salt (KBR) solution (pH 7.6) containing 8.4 mM
glucose. The flasks are gassed with O2:CO2 (95%:5%),
tightly closed and placed in a shaker/water bath (37°C) for incubation of the
fragments. The incubation media are replaced with fresh KBR after preincubation
for 1 h. The ovaries are further incubated for 4 h in the presence or absence
of inhibitors. 4-OH androstendione is used as standard in concentrations
between 0.33 and 330 μM/l. At the end of the experiment,
the incubation media are removed and centrifuged. In the supernatant oestrogen,
progesterone and testosterone are determined by radioimmunoassays. The data of
control and test group are compared with suitable statistical analysis.
Screening Methods for Progestins:
In
Vivo Methods
Proliferation
of uterine endometrium in oestrogen-primed rabbits: Clauberg–McPhail test
Female rabbits weighing 800–1000 g are primed with
estradiol. They are then administered with progestational compounds leading to
the proliferation of endometrium and converted into the secretary phase. This
principle is used for the screening of progestational compounds.
Female rabbits weighing 800–1000 g are primed with a daily
injection of oestradiol 0.5 μg/ml in aqueous solution. On day 7,
the drug treatment is begun. The total dose is given in five equally divided
fractions daily over five days. Twenty-four hours after the last injection, the
animals are killed. The uteri are dissected out, and frozen sections of the
middle portion of one horn is prepared and examined for histological
interpretation. For interpretation of pro-gestational proliferation of
endometrium, the beginning of glandular development may be graded 1 and
endometrium consisting only of glandular tissue may be graded 4.
Pregnancy
maintenance test
Progesterone is responsible for the maintenance of
preg-nancy. This principle is used for the screening of proges-tational
compound.
Ovariectomy is done on day 5/10/15 day of pregnancy in different
groups of pregnant rats. The animals are treated with different test and
standard drugs. Pregnant rats are killed 5/10/15 days later. An average of
living foetuses at the end of the experiment is compared with the standard and
the control group (without ovariectomy). The ED50 of progesterone is
5 mg/day in rat and less than 0.5 mg/ day in mouse.
Carbonic
anhydrase activity in rabbit’s endometrium
There is a linear dose-response relationship between dose of
progestogens and carbonic anhydrase activity in rabbit endometrium. This
principle is used for the screening of progestational compounds.
Immature female albino rabbits are used in this study. The
animals are primed with estradiol and administered test and standard drugs.
After the drug treatment, the animals are sacrificed and their uterus removed.
The endometrial extract of the uterus is evaluated for the carbonic anhydrase
activity calorimetrically.
Prevention
of abortion in oxytocin-treated pregnant rabbits
Administration of oxytocin by i.v. route to pregnant rabbits
on the 30th day of pregnancy causes abortion. Prior admin-istration of
progestational compounds prevents the abortion. This principle is used for the
detection and screening of progestational compounds.
Ten units of oxytocin are administered to pregnant rabbits
on day 30 of pregnancy. Test and standard drugs in oil are injected 24 hours
before. The control animal not receiving any drugs aborts within 2–30 min after
administration of oxytocin. Drugs which have progestational activity prevent
abortion.
Deciduoma
reaction in rats
This study is based on the phenomenon of maternal/placen-tal
tumour formation due to progestational drugs in trau-matized uterus of
ovariectomized rats. This phenomenon is used for the screening of progestational
compounds.
Ovariectomized adult female albino rats weighing between 150
and 200 g are used for the study. The rats are primed with four injections of 1
μg oestrone. This is followed by nine days of drug therapy.
On day five, one uterine horn is exposed and 1 mg of histamine dihydro-chloride
injected into the lumen. Twenty-four hours after the last dose of drug, the
animals are killed, the uterine horn cut off, weighed, and histologically
examined.
Screening Methods for Progestins; In Vitro Methods
Progesterone
receptor-binding assay
Progesterone receptor-binding assay uses the principle of
competitive binding of labelled and unlabelled progester-one on progesterone
receptors. Progestational compounds displace the labelled progesterone in a
concentration-de-pendent manner from the progesterone receptor.
Human uteri obtained after hysterectomy is frozen in liquid
nitrogen and stored at 80°C until use. For cytosol preparation, uterine tissues
are minced and homogenized with a homogenizer at 0–4°C in ice-cold PENG buffer
composed of 10 mM KH2PO4, 10 mM K2HPO4,
1.5 mM EDTA, 3 mM NaN3, 10% glycerol, pH 7.5. The homoge-nates are
then centrifuged at 10,5000 g at 4°C for 30 min. The supernatant is taken as
cytosol.
The cytosol preparations are incubated with 3H-R5020
as radio-ligand at a concentration of 8 nmol/l and increased concentrations (1
× 10–10 to 1 × 10–5 mol/l) of the competi-tor steroid
overnight at 4°C. Then unbound steroids are adsorbed by incubating with 0.5 ml
of DCC (0.5% carbon (Norit A), 0.05% dextran T400 in PENG buffer) for 10 min.
at 4°C. After centrifugation (10 min at 1,500 g at 4°C), 0.5 ml of the
supernatant is withdrawn and counted for radioactivity. To calculate the
relative binding affinity, the percentage of radio-ligand bound in the presence
of the competitor compared to that bound in its absence is plotted against the
concentration of unlabelled competing steroid.
Screening Method for Antiprogestational Activity
Antagonism
of physiological effect of progesterone
The antiprogestational compound inhibits some or all the
physiological effect of progesterones. This principle is used to screen the
antiprogestational activity of drugs.
The procedures for assay of the Clauberg–McPhail test and
deciduoma formation are as described for progestational activity except that
the test compounds are given along with the progesterone.
Screening Method for Antiimplantation Activity
Female albino rats of established fertility in the
proestrous or estrous stage are mated with mature male rats of estab-lished
fertility (in the female : male ratio of 3:1). Each female is examined for the
presence of spermatozoa in the early morning vaginal smear. The day on which
this sign of mating is seen is taken as day 1 of pregnancy. The female is then
separated and caged singly. The test drug is administered orally to the
animals once daily on specific days of pregnancy at different concentrations.
On day 10th of pregnancy, the animals are laparotomized, and the number of
implants present in both the uterine horns as well as the number of corpora
lutea (CL) on each ovary is counted. The animals are allowed to complete the
gestation period (usually 21–23 days) and the number of litters delivered, if
any are counted. Preimplantation loss and postimplantation loss are calculated
using the following formula.
Preimplantation loss = No. of CL on 10th day—No. of implants
on 10th day
Post implantation loss = No. of implants on 10th day—No. of
litters delivered
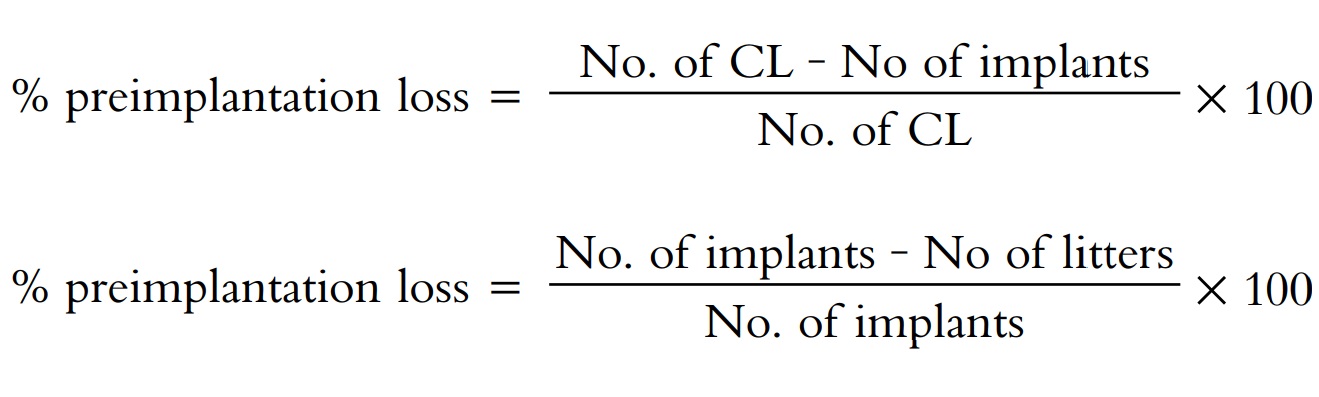
The animal is anaesthetized with ether and the limbs tied to
a rat board (waxed) with the ventral side up. The hairs on the area around the
midline abdominal region are clipped with a curved scissor and the region
cleaned with 70% alcohol. An incision of 2 cm length is made along the midline
to expose the viscera. The superficially tying coils of ileum are lifted to
expose the two uterine horns. The horns are examined for implantation sites.
Implants are visible as clear swellings on the uterine horns giving the uterine
tube a beaded appearance. Embryos with a bright red dish aspect and a clear
margin are considered to be healthy. Those of a dull blue colour with no clear
margin and orientation with some exudates are considered resorbing. The number
of implants and resorption sites per horn are counted. The ovaries, which lie
on the upper end of the uterine horns, show corpora lutea as yellow spots over
the surface. The number of corpora lutea present on each ovary is also noted.
After counting, the organs are replaced back. A small
quantity of neosporin powder is sprinkled over the organs to prevent any
infection. The incision through the muscular layer is closed with a continuous
suture using absorbable catguts. The skin layer is closed with continuous
sutures using silk thread. An antiseptic, povidone iodine solution, is applied
on the sutured area after wiping with 70% alcohol. The animal is maintained on
light ether anaes-thesia throughout the experiment. After laparotomy, the rats
are transferred to a warm place till they recover from the anaesthesia.
Screening Methods for Abortifacient Activity
Adult female albino rabbits are used for the study. The
pregnancy date is counted from the date of observed mating. The existence of
pregnancy may be confirmed by palpation after the 12th day of pregnancy.
Intra-amniotic and intra-placental injections are administered to the rabbits
under ether anaesthesia on the 20th day of pregnancy. The uterus is exposed
through a midline incision, its various parts are identified by
transillumination from a strong source of light and a particular site chosen
for injection. Then the material is injected in 0.l ml of solvent into the
amniotic fluid or in 0.05 ml of solvent into the placenta.
Alternatively, the drugs can be given through any route and
duration from the 20th day of pregnancy. The effect of the drug is determined
by looking for vaginal bleeding, changes in weight, abdominal palpation, and by
postmor-tem examination.
Screening Methods for Antifertility Activity in
Males
Developing male antifertility agent involves interference
with spermatogenesis without loss of libido and varying sexual characteristics.
The general approaches include:
1. Emergent spermatozoa made
nonfunctional
2. Production of oligospermia/aspermia
In
vivo methods
Fertility test: Fertility test is based on the
evaluation of the average litter size. Antifertility agents negatively
affect the average litter size.
Groups of 5–10 male rats of proven fertility are treated
with the drug and are paired with fertile females in the ratio of 1:3. Daily
vaginal smears are examined for the presence of sperms; normally within one
week all females which have passed through one estrus cycle would have mated.
The mated animals are kept separately till the gestational period. The litters
are counted and using the following formula; the average litter size is
calculated.
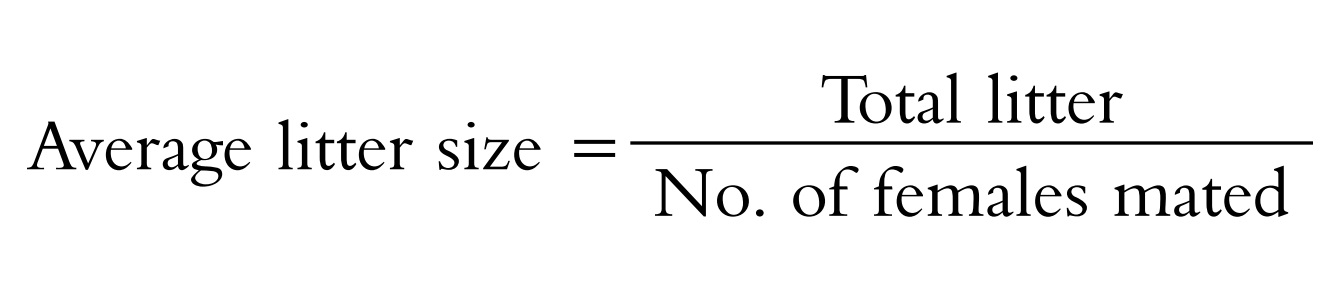
If vaginal smear shows leucocytes in 10–14 days,
pseudopregnancy is confirmed. If insemination is not detected then inhibition
of libido or aspemic copulation might be the cause. Fertility patterns can be
obtained from changes in average litter size.
Cohabitation test: This test determines the time
interval for litter
production after placing treated males with two females each. The date of
mating is calculated from the date of parturition. This method is suitable for
drugs known to cause sterility for several weeks.
Adult female and male albino rats of proven fertility are
used for the study. They are kept for mating in the ratio of 2:1 till both
females deliver litters. The date of mating is calculated from the date of
parturition. The time interval for litter production after placing treated
males with the two females is calculated.
Subsidiary test: This test determines the changes in
sper-matozoa count with time. The antifertility drugs affect the spermatozoa
count negatively.
Adult male albino rats weighing between 150 and 250 g are
used for the study. They are kept in a cage containing artificial or animal
vagina. The vagina is artificially stimu-lated by a cylindrical plastic jacket
with a rubber liner filled with water at 5°C. About 0.5 ml of ejaculate is
diluted with saline containing traces of formalin. The resulting suspen-sion is
counted on a haemocytometer.
In
vitro methods
Spermicidal activity: Spermicidal drugs are diluted with normal saline, and serial dilutions
are made. About 0.2 ml of human seminal fluid is mixed with 1 ml of spermicidal
solution. Then the mixture is incubated at 37°C for 30 min. A drop of the
mixture is placed immediately on a slide, and at least five fields are
microscopically observed under high power (×400) for assessment of sperm
morphologi-cal changes and motility. Effective agents can immobilize and kill
the sperms.
Immobilization assay: The cauda portion of the epididy-mes
of a ram is isolated and minced in 0.9% saline solution (pH 7.5). It is
filtered through a piece of cheese cloth to get a sperm suspension. For human
samples, ejaculates (n = 10) from normal subjects after 72–96 h of sexual
absti-nence are subjected to routine semen analysis following liquefaction at
37°C. Sperm count above 100 million/ml and viability above 60% with normal
morphology and rapid and progressive motility is employed for the test.
Ram epididymal sperm suspension (100–200 million/ ml) or
human ejaculate (100–150 million/ml) are mixed thoroughly in a 1:1 ratio with
different concentration of drugs. A drop of the mixture is placed immediately
on a slide and at least five fields are microscopically observed under high
power (×400) for assessment of sperm motility. The mixture is then incubated at
37°C for 30 min, and the above process is repeated.
Nonspecific aggregation estimation: Different concen-trations of drugs
are treated with ram sperm suspension in a 1:1 ratio and kept at 37°C for 1 h.
One drop of the sedimented sperm is then taken from the bottom of the micro
centrifuge tube, placed on a slide, and the percent aggregation examined
microscopically under 400× mag-nifications. Since the nonaggregated spermatozoa
remain in the supernatant, the latter is collected and the turbidity determined
spectrophotomctrically at 545 nm. The aggrega-tion is indirectly proportional
to the sperm viability.
Sperm revival test: This assay determines the extent of spermicidal and immobilization
capability of drugs by evaluating the revival of sperm motility.
To study the revival of sperm motility, after completion of
the immobilization assay, the spermatozoa are washed twice in physiological
saline. They are then incubated once again in the same medium free of drug at
37°C for 30 min to observe the reversal of sperm motility.
Assessment of plasma membrane integrity: To assess the sperm plasma membrane, integrity ram sperm sus-pension
(100–200 million/ml) or human ejaculated sperm (100–150 million/ml) are mixed
with the drug at the minimum effective concentration, at a ratio of 1:1 and
incubated for 30 min at 37°C. Sperm samples mixed with saline in a similar
manner serve as controls. For viability assessment, one drop each of 1% aqueous
solution of eosin Y and of 10% aqueous solution of nigrosin was placed in a
micro centrifuge tube. A drop of well-mixed sperm sample is added to it and
mixed thoroughly. The mixture is dropped onto a glass slide and observed under
400x magnification.
For the hypoosmotic swelling test (HOS), 0.1 ml of aliquot
is taken from each of the treated and control sample, mixed thoroughly with 1
ml of HOS medium (1.47% fruc-tose and 2.7% sodium citrate at a 1:1 ratio) and
incubated for 30 min. at 37°C. The curling tails are examined under phase
contrast microscope using l00x magnification.
5-Nucleotidase is released possibly due to destabilization
of plasma membrane. This can be estimated to determine the effect of the drug
on the plasma membrane integrity of the sperm. The activity of 5’-nucleotidase
can be determined by measuring the rate of release of inorganic phosphate from
adenosine 5’-monophosphate. After incubating the sperm suspension with the
drug, the sperm pellet is collected by centrifugation at 3,000 g at 37°C. It is
then washed twice in 0.9% saline and suspended in 0.1 mol/l Tris-HCl buffer (pH
8.5) with each reaction system containing (100–200) million spermatozoa. An
aliquot of 0.1 ml suspension of sperm is added to 0.9 ml of buffered substrate
containing 3 mmol/l adenosine 5’-monophosphate and 50 mmol/1 MgCl2
dissolved in 0.1 mol/1 Tris-HCl buffer. The tubes are incubated at 37°C for 30
min, and 0.5 ml 20% TCA (0–4°C) is added to the mixture to stop the reaction.
The mixture is then centrifuged at
10,000 g at 4°C. The pellet is discarded and the supernatant kept for phosphate
estimation. The activity of 5’-nucleotidase is expressed in terms of μg of phosphate released. The activity of 5’-nucleotidase is indirectly proportional to the plasma membrane integrity.
Evaluation of acrosomal status: This method evaluates the acrosomal status of sperm. The
acrosome is the cap-like structure on the head of the spermatozoa. It breaks
down just before fertilization, releasing a number of enzymes that assist
penetration between the follicle cells that surround the ovum. The most widely
studied acrosomal enzyme is the acrosin that has been shown to be associated
with acrosoraes of all mammalian spermatozoa. The highest substrate specificity
was obtained with BAEE (N-benzoyl-L-argine ethyl ester).
Different concentrations of drugs are mixed with ram sperm
suspension in a 1:1 ratio and kept at 37°C for 1 h. The suspension is
centrifuged and the pellets collected. The pellets are extracted with 3 μmol/l HCl at pH 3 and the enzyme activity is measured,
following the hydrolysis of 0.5 μmol/l BAEE dissolved in 0.05 mol/1
Tris-HCl buffer containing 0.05 mol/1 CaCl2 at pH 8. The activity of
acrosin is expressed in terms of mIU. One mIU activity means the amount of
enzyme, which causes the hydrolysis of one nano mole of BAEE in 1 min at 25°C.
The activity of acrosin is directly proportional to the fertilizing capability
of sperms.
Androgenic and antiandrogenic activities: Androgenic compounds increase the weight of the
testes and seminal vesicles of immature male mice. Antiandrogenic compounds
suppress the increase in weight responses of testosterone. This principle is
used for the screening of androgenic and antiandrogenic activity.
Immature male mice weighing around 20 g are used for the
study. The drugs are administered for seven days alone and along with
testosterone. Twenty-four hours after the last dose, the animals are weighed
and sacrificed with an over dose of ether. The testes and seminal vesicles are
removed and weighed rapidly in a sensitive balance. The weights are compared
with the control group.
Related Topics
