Screening Methods for Antiinflammatory Agents
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : Biological Screening of Herbal Drugs
WHO has identified 2000–2010 as the decade for musculoskeletal disorders. Herbal drugs like holy basil (tulsi; Ocimum sanctum), turmeric (Curcuma longa), Indian olibanum tree (Boswellia serrata), ginger (Zingiber officnale), etc. are widely used for the treatment of various inflammatory disorders.
SCREENING METHODS FOR ANTIINFLAMMATORY AGENTS
WHO has identified 2000–2010 as the decade for
musculoskeletal disorders. Herbal drugs like holy basil (tulsi; Ocimum sanctum), turmeric (Curcuma longa), Indian olibanum tree (Boswellia serrata), ginger (Zingiber officnale), etc. are widely used
for the treatment of various inflammatory disorders. They are not only found to
be safer and have fewer side effects, but they also cover a large domain of
mechanisms involved in inflammation thus proving to be more beneficial than
synthetic drugs. Inflammation expresses the response to damage of cells and
vascular tissues. The five basic symptoms of inflammation—redness, swelling,
heat, pain, and deranged function, have been known since the ancient Greek and
Roman era.
The major events occurring during this response are an
increased blood supply to the affected tissue by vasodila-tion, increased
capillary permeability caused by retraction of the endothelial cells which
allows the soluble mediators of immunity to reach the site of inflammation and
leukocytes migration out of the capillaries into the surrounding tissues.
Neutrophils, monocytes, and lymphocytes also migrate towards the site of
infection. The development of inflam-matory reactions is controlled by the
following systems: cytokines, complement, kinin and fibrinocytic pathways; by
lipid mediators (prostagiandins and leukotrienes) released from different
cells; and by vasoactive mediators released from mast cells, basophils, and
platelets.
The response is accompanied by the clinical signs of erythema,
oedema, hyperalgesia, and pain. Inflammatory responses occur in three distinct
phases, each apparently mediated by different mechanisms:
Acute transient phase: Characterized by local
vasodilatation and increased
capillary permeability.
Sub-acute phase: Characterized by infiltration of
leuko-cytes and phagocytic cells.
Chronic proliferative phase: Tissue degeneration and fibrosis occur.
Drugs preventing acute and sub-acute inflammation can be
tested using the following models: paw oedema in rats, croton oil ear oedema,
pleurisy tests, UV-erythema in guinea pigs, oxazolone-induced ear oedema in
mice, granuloma pouch technique, and vascular permeability. The effectiveness
of drugs which work at the proliferative phase can be measured by methods for testing
granuloma formation, such as the cotton pellet granuloma, adjuvant-induced
arthritis, glass rod granuloma, and PVC sponge granuloma.
Testing of Drugs Preventing Acute and Sub-Acute
Inflammation
Paw
oedema
This technique is based upon the ability of antiinflammatory
agents to inhibit the oedema produced in the hind paw of the rat after
injection of a phlogistic agent (irritant). Rats with a body weight between 100
and 150 g are required. Many irritants have been used, such as brewer’s yeast,
formaldehyde, dextran, egg albumin, kaolin, Aerosil®, and sulphated
polysaccharides like carrageenan. The animals are fasted overnight. The control
rats receive distilled water while the test animals receive drug suspension
orally. Thirty minutes later, the rats are subcutaneously injected with 0.1 ml
of 1% solution of carrageenan in the foot pad of the left hind paw. The paw is
marked with ink and immersed in the water cell of a plethysmometer up to this
mark. The paw volume is measured plethysmographically immediately after
injection, 3 and 6 h after injection, and eventually 24 h after injection. The
paw volumes for the control group are then compared with those of the test
group.
Croton
oil ear oedema in rats and mice
This method mainly evaluates the antiphlogistic activity of
topically applied steroids.
For this method, mice (22 g) or rats (70 g) are required.
For tests in mice, the irritant is composed of (v/v): 1 part croton oil, 10
parts ethanol, 20 parts pyridine, and 69 parts ethyl ether; for rats the irritant
is composed of (v/v): 4 parts croton oil, 10 parts ethanol, 20 parts pyridine,
and 66 parts ethyl ether. The standard and the test compound are dissolved in
this solution. Irritants are applied on both sides of the right ear (0.01 ml in
mice or 0.02 ml in rats under ether anaesthesia). Controls receive only the
irritant solvent. The left ear remains untreated. Four hours after application,
the animals are sacrificed under anaesthesia. Both ears are removed and discs
of 8 mm diameter are cut. The discs are weighed immediately and the weight
difference between the treated and untreated ear is recorded indicating the
degree of inflammatory oedema.
Pleurisy
test
Pleurisy is the phenomenon of exudative inflammation in man.
In experimental animals, pleurisy can be induced by several irritants, such as
carrageenan, histamine, bradyki-nin prostaglandins, mast cell degranulators,
and dextran. Leukocyte migration and various biochemical parameters involved in
the inflammatory response can be measured easily in the exudate.
Male rats weighing 220–260 g are required. The animal is
lightly anaesthetized with ether and placed on its back. The hair from the skin
over the ribs on the right side is removed and the region cleaned with alcohol.
A small inci-sion is made into the skin under the right arm. The wound is
opened and 0.1 ml of 2% carrageenan solution is injected into the pleural
cavity through this incision. The wound is closed with a clip. One hour before
this injection and 24 and 48 h thereafter, rats are treated (subcutaneously or
orally) with the standard or the test compound. A control group receives only
the vehicle. The animals are sacrificed 72 h after carrageenan injection and
pinned on a dissec-tion board with the forelimbs fully extended. About 1 ml of
heparinized Hank’s solution is injected into the pleural cavity through an
incision. The cavity is gently massaged to mix its contents. The fluid is
aspirated out of the cavity using a pipette. The aspirated exudates are
collected in a graduated plastic tube. About 1 ml (the added Hank’s solu-tion)
is subtracted from the measured volume. The values of each experimental group
are averaged and compared with the control group. The white blood cell number
in the exudate is measured using a Coulter counter or a haematocytometer.
Ultraviolet
erythema in guinea pigs
Antiinflammatory agents delay the development of
ultra-violet erythema on albino guinea pigs. They are shaved on the back 18 h
before testing. The test compound is suspended in the vehicle and half the dose
of the test compound is administered orally 30 min before ultraviolet exposure.
Control animals are treated with the vehicle alone. The guinea pigs are placed
in a leather cuff with a hole of 1.5–2.5 cm size punched in it, allowing the
ultraviolet radiation to reach only this area. An ultraviolet burner is warmed
up for about 30 min before use and placed at a constant distance (20 cm) above
the animal. Following a 2 min ultraviolet exposure, the remaining half of the
test compound is administered. The erythema is scored 2 h and 4 h after
exposure.
Oxazolone-induced
ear oedema in mice
The oxazolonc-induced ear oedema in mice is a model of
delayed contact hypersensitivity that permits the quantita-tive evaluation of
the topical and systemic antiinflammatory activity of a compound following
topical administration.
Mice of either sex (25 g) are required. A fresh 2% solu-tion
of oxazolone in acetone is prepared. This solution (0.01 ml) is injected on the
inside of both ears under anaesthesia. The mice are injected 8 days later,
again under anaesthesia, with 0.01 ml of 2% oxazolone solution (control) or
0.01 ml of oxazolone solution in which the test compound or the standard is
dissolved, on the inside of the right ear. The left ear remains untreated. The
maximum of inflammation occurs 24 h later. At this time the animals are
sacrificed under anaesthesia and a disc of 8 mm diameter is punched from both
ears. The discs are immediately balance. The weight difference is an indicator
of the inflammatory oedema.
Granuloma
pouch technique
Irritants such as croton oil or carrageenan produce aseptic
inflammation resulting in large volumes of exudate, which resembles the
sub-acute type of inflammation. Rats (150–200 ) are selected for the study; the
back of the animals is shaved and disinfected. With a very thin needle, an air
pouch is made by injection of 20 ml of air under ether anaesthesia. Into the
resulting air pouch 0.5 ml of a 1% solution of croton oil in sesame oil is
injected. After 48 h, the air is withdrawn from the pouch and 72 h later any
resulting adhesions are broken. Instead of croton oil, 1 ml of a 20% suspension
of carrageenan in sesame oil can be used as irritant. Starting with the
formation of the pouch, the animals are treated every day either orally or
subcuta-neously with the test compound or the standard. On the fifth day, the
animals are sacrificed under anaesthesia. The pouch is opened and the exudate
collected in glass cylinders. The average value of the exudate of the controls
and the test groups is calculated.
Vascular
permeability
This test is used to evaluate the inhibitory activity of
drugs against increased vascular permeability, which is induced by a phlogistic
substance. Mediators of inflammation, such as histamine, prostaglandins, and
leucotrienes are released following stimulation of mast cells. This leads to a
dila-tion of arterioles and venules and to an increased vascular permeability.
As a consequence, fluid and plasma proteins are released and edemas are formed.
Vascular permeability is increased by subcutaneous injection of the mast
cell-degranulating compound 48/80. The increase of perme-ability can be
recognised by the infiltration of the injected sites of the skin with the dye
Evan’s blue.
Male rats (160 and 200 g) are used. About 5 ml/kg of 1%
solution of Evan’s blue is injected intravenously. One hour later, the animals
are dosed with the test compound orally or intraperitoneally. After 30 min, the
animals are lightly anaesthetized with ether and 0.05 ml of 0.01% solution of
compound 48/80 is injected subcutaneously at three sites. About 90 min after
the injection of compound 48/80, the animals are sacrificed by ether
anaesthesia. The abdominal skin is removed and the dye-infiltrated areas of the
skin measured. The percent inhibition in the treated animals as compared to the
control group is calculated.
Testing of Drugs Preventing the Proliferative
Phase (Granuloma Formation) of Inflammation
Cotton
pellet granuloma
Foreign body granulomas are induced in rats by the
subcu-taneous implantation of pellets of compressed cotton. After several days,
histologically giant cells and undifferentiated connective tissue can be
observed besides fluid infiltra-tion. The amount of newly formed connective
tissue can be measured by weighing the dried pellets after removal. More
intensive granuloma formation has been observed if the cotton pellets are
impregnated with carrageenan.
Male and female rats with an average weight of 200 g are
used. The back skin is shaved and disinfected with 70% ethanol. An incision is
made in the lumbar or neck region. Subcutaneous tunnels are formed and a
sterilized cotton pellet is placed with the help of a blunted forceps. The
animals are treated for seven days subcutaneously or orally. They are then sacrificed,
the pellets taken out and dried. The net dry weight, that is, after subtracting
the weight of the cotton pellet is determined. The average weight of the
pellets of the control group as well as that of the test group is calculated.
The percent change of granuloma weight relative to the vehicle control group is
determined.
Adjuvant
arthritis in rats
Adjuvant-induced arthritis in rats exhibit many similarities
to human rheumatoid arthritis. An injection of complete Freund’s adjuvant into
the rat’s paw induces inflammation as a primary lesion with a maximum
inflammation after three to five days. Secondary lesions occur after a delay of
approximately 11 to 12 days and are characterized by inflammation of
noninjected sites (hind legs, forepaws, ears, nose, and tail), a decrease in
weight and immune responses.
Male rats with an initial body weight of 130 to 200 g are
used. On day 1, rats are injected in the sub-plantar region of the left hind
paw with 0.1 ml of complete Freund’s adjuvant. The adjuvant consists of 6 mg mycobacterium butyricum thoroughly ground with a mortar and pestle and suspended in heavy paraffin oil (Merck)
to give a concentration of 6 mg/ml. Dosing with the test compounds or the
standard is started on the same day and continued for 12 days. Both paw volumes
and body weight are recorded on the day of injection. The paw volume is
measured plethysmographically with equipment as described in the paw oedema
tests. On day 5, the volume of the injected paw is measured again, indicating the
primary lesion and the influence of therapeutic agents on this phase. The
severity of the induced adjuvant disease is determined by measuring the
noninjected paw (secondary lesions) with a plethysmometer. The animals are not
dosed with the test compound or the standard from day 12 to 21. On day 21, the
body weight is determined again and the severity of the secondary lesions
evaluated visually and graded according to the following scheme:
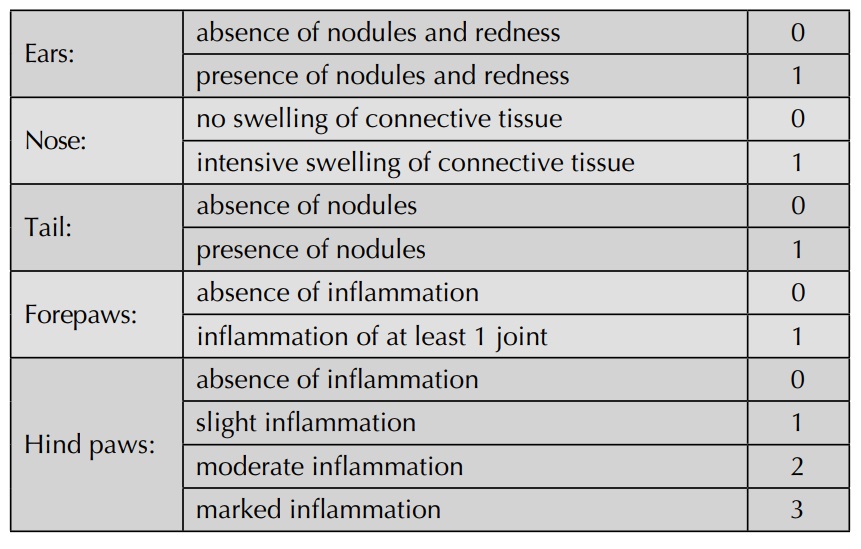
Sponge
implantation technique
Foreign body granulomas are induced in rats by subcutane-ous
implantation of a sponge. Sponges used for implanta-tion are prepared from
polyvinyl foam sheets (thickness: 5 mm). Discs are punched out to a standard
size and weight (10.0 ± 0.02 mg). The sponges are then soaked in 70% v/v
ethanol for 30 min., rinsed four times with distilled water, and healed at 80°C
for 2 h. Before implantation in the animal, the sponges are soaked in sterile
0.9% saline in which either drugs, antigens, or irritants have been sus-pended.
Typical examples include 1% carrageenan, 1% yeast, 1% zymosan A, 6% dextran,
heat-killed Bordctelhi pertussis, or
0.5% heat-killed Mycobacterium
tuberculosis.
Sponges are implanted in rats weighing 150–200 g under ether
anaesthesia. An incision is made and separate cavities are formed into which
sponges are inserted. Up to 8 sponges may be implanted per rat. The incision is
closed with Michel clips and the animals maintained at a constant temperature
of 24°C. For short-term experiments, the animals are treated with the test drug
or standard once before implantation orally or subcutaneously. For long-term
experiments, the rats are treated daily up to 3 weeks.
Glass
rod granuloma
Glass rod-induced granulomas reflect the chronic proliferate
phase of inflammation. Of the newly formed connective tissue, not only can the
wet and dry weight be measured, but also the chemical composition and
mechanical proper-ties. Glass rods with a diameter of 6 mm are cut to a length
of 40 mm and the ends rounded off. They are sterilized before implantation.
Rats are anaesthetized with ether, the back skin shaved and disinfected. From
an incision in the back region, a subcutaneous tunnel is formed with a blunted
forceps. A glass rod is introduced into this tunnel. The incision wound is
closed by sutures. The animals are kept in separate cages. The rods remain in
situ for 20 or 40 days. Animals are treated orally. At the end of 20 days the
animals are sacrificed. The glass rods are removed together with the surrounding
connective tissue, which forms a tube around the glass rod. By incision at one
end, the glass rod is extracted and the granuloma sac inverted forming a plain
piece of pure connective tissue. Wet weight of the granuloma tissue is
recorded. The specimens are kept in a humid chamber until further analysis.
Biochemical analyses, such as determination of collagen and glycosaminoglycans,
can also be performed.
Related Topics
