Biosynthesis of Alkaloids
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : General Biosynthetic Pathways of Secondary Metabolites
The biosynthesis of different groups of alkaloids has now been investigated to some extent using precursors labelled with radioactive atoms. Very little work has, however, been published in the area of enzymology of alkaloid bio-synthesis, some exceptions being in studies of ergot and Amaryllidaceae alkaloids. The biosynthetic pathways of pharmacognostically important alkaloids are given here.
BIOSYNTHESIS OF ALKALOIDS
The biosynthesis of different groups of alkaloids has now been investigated to some extent using precursors labelled with radioactive atoms. Very little work has, however, been published in the area of enzymology of alkaloid bio-synthesis, some exceptions being in studies of ergot and Amaryllidaceae alkaloids. The biosynthetic pathways of pharmacognostically important alkaloids are given below.
Ornithine Derivatives
L-Ornithine is a nonprotein amino acid forming part of the urea cycle in animals, where it is produced from L-arginine in a reaction catalysed by the enzyme arginase. In plants it is formed mainly from L-glutamate. Orni-thine contains both δ- and α-amino groups, and it is the nitrogen from the former group which is incorporated into alkaloid structures along with the carbon chain, except for the carboxyl group. Thus ornithine supplies a C4N building block to the alkaloid, principally as a pyrrolidine ring system, but also as part of the tropane alkaloids (Figure below).
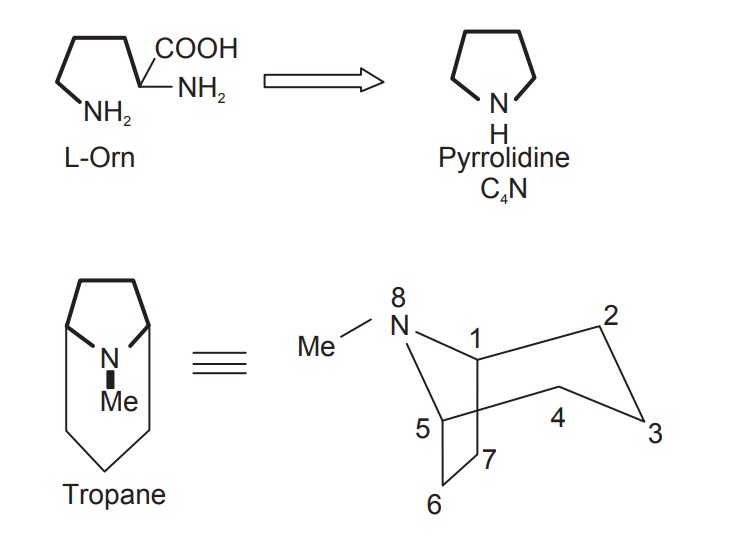
Ornithine is a precursor of the cyclic pyrrolidines that occur in the alkaloids of tobacco (nicotine, nornicotine) and in solanaceae family. Most of the tobacco alkaoids have nicotine as the starting compound. Few of the intermediates produced during the biosynthesis of tropane are also the starting compounds for hyoscamine and cocaine.
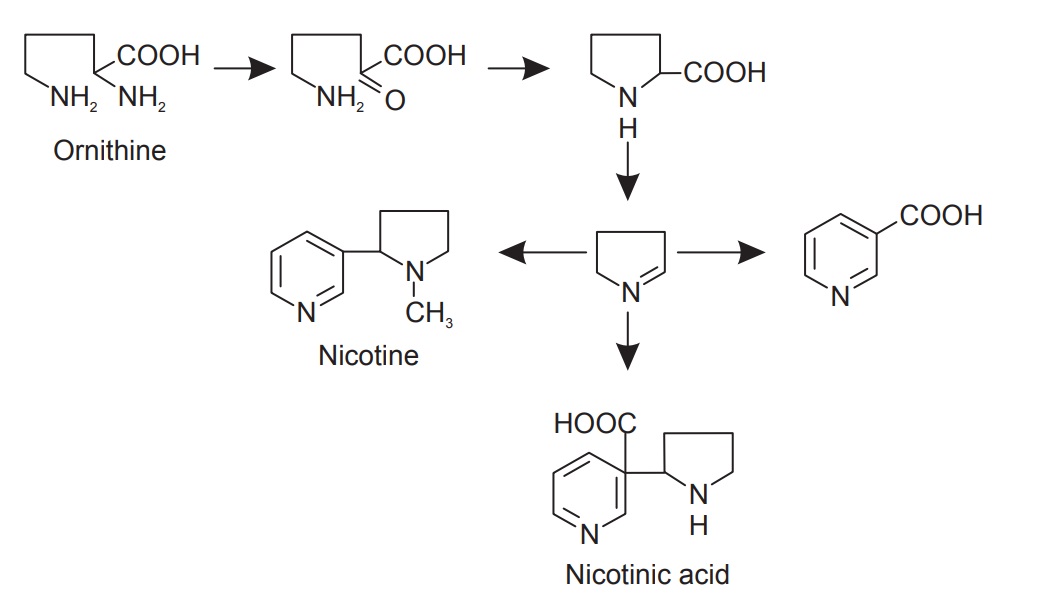
Biosynthesis of tropane
The starting compound of this synthesis is ornithine and methylornithine is the first intermediate.
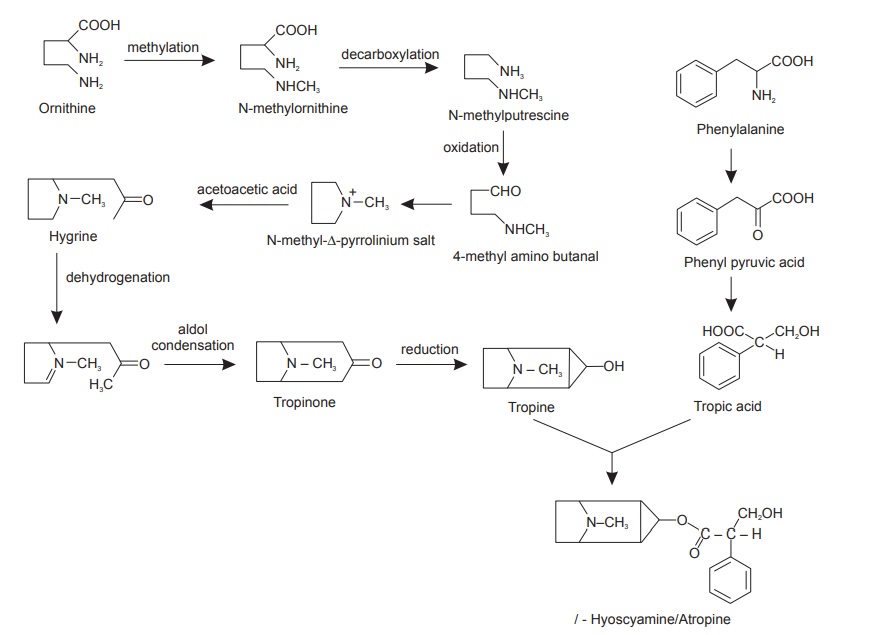
Biosynthesis of atropine
Lysine Derivatives
L-Lysine is the homologue of L-ornithine, and it too functions as an alkaloid precursor, using pathways analogous to those noted for ornithine. The extra methylene group in lysine means this amino acid participates in forming six-membered piperidine rings, just as ornithine provided five-membered pyrrolidine rings. As with ornithine, the carboxyl group is lost, the ε-amino nitrogen rather than the α-amino nitrogen is retained, and lysine thus supplies a C5N building block (Figure below).
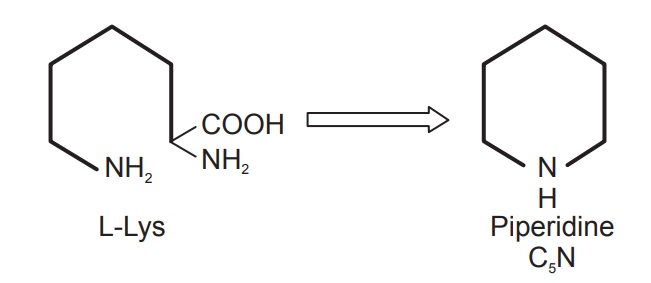
Lysine is a precursor for piperidine. Piperidine forms the basic skeleton for numerous alkaloids. Lysine and its derivatives are responsible for the biogenesis of some of the bitter principles of the lupine, lupinine, lupanine, ana-basine, pelletierine, and some other alkaloidal compounds. Lycopodium, a substance obtained from Lycopodium spp., also belongs to this group.
Phenylalanine Derivatives
Whilst the aromatic amino acid L-tyrosine is a common and extremely important precursor of alkaloids, L-phenylalanine is less frequently utilized, and usually it contributes only carbon atoms, for example, C6C3, C6C2, or C6C1 units, without providing a nitrogen atom from its amino group. Ephedrine (Figure (c)), the main alkaloid in species of Ephedra (Ephedraceae) and a valuable nasal decongestant and bronchial dilator, is a prime example.
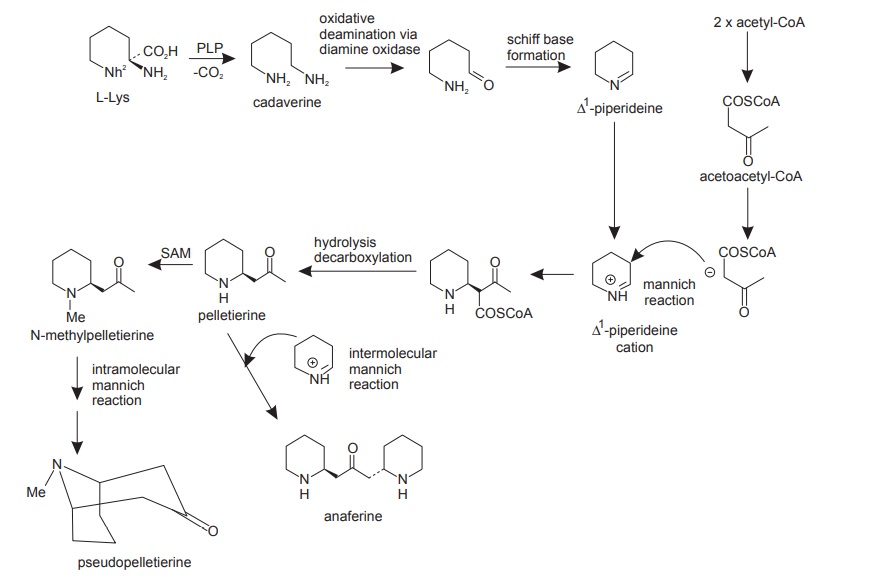
(a) Biosynthesis of pseudopelletierine
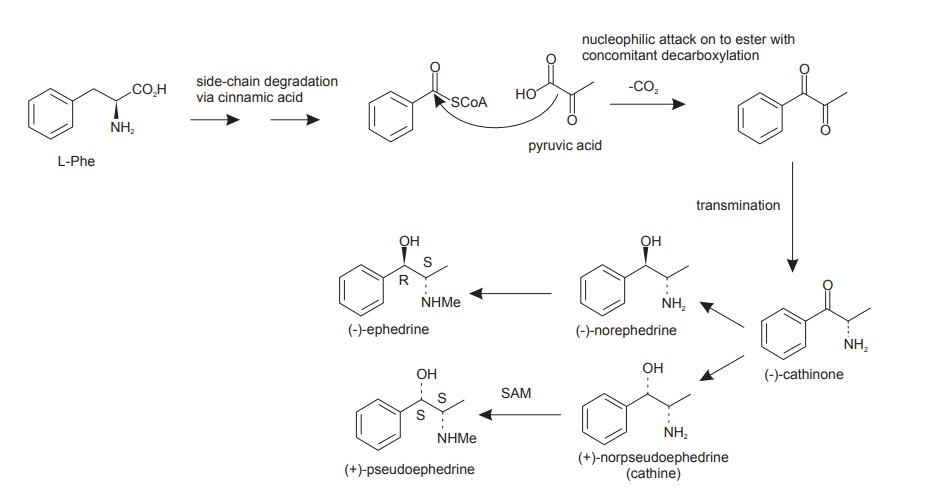
Tyrosine Derivatives
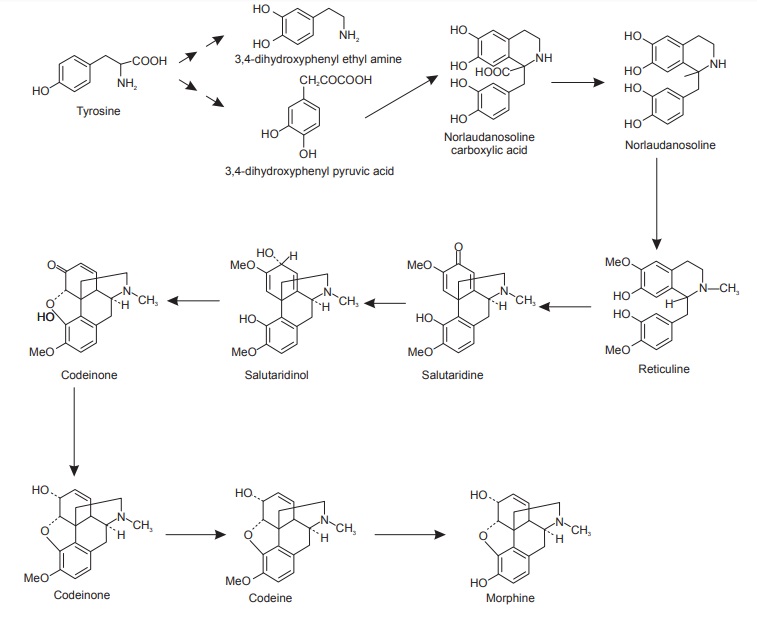
The first essential intermediate is dopamine; dopamine is the precursor in the biosynthesis of papaverine, berberine, and morphine. Tyrosine is considered to be a precursor for the huge family containing alkaloids.
Biosynthesis of morphine
A range of similar compounds like the opium alkaloids, thebaine, codeine, etc. are derived during the formation of morphine.
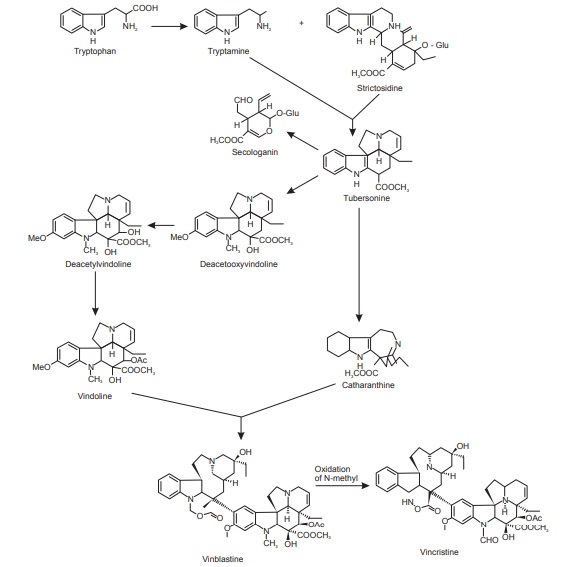
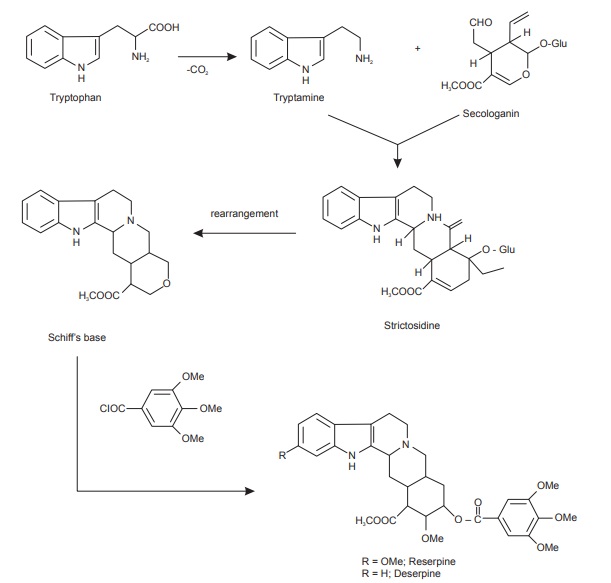
Tryptophan Derivatives
About 1,200 dissimilar compounds, the entire of which are tryptophan derivatives have been isolated till today. The tryptophan derivatives correspond to 25% of all known alkaloids and many of them are medicinally valuable. Tryptophan and its decarboxylated product (tryptamine) are precursors for the biosynthesis of broad range of indole alkaloids of which the vinca and rauwolfia alkaloids are examples; and also in the alkaloids belonging to families like Apocynaceae, Loganiaceae, and Rubiaceae. D-Tubocurarine, the active components of curare, is also a tryptophan derivative. Tryptamine on condensation with secologanin produces vincoside a nitrogenous glucoside. Some of the indole alkaloids in vinca are formed from vincoside.
Biosynthesis of quinoline alkaloids
Some of the most remarkable examples of terpenoid indole alkaloid modifications are to be found in the genus Cinchona (Rubiaceae), in the alkaloids quinine, quinidine, cinchonidine, and cinchonine (Figure below), long prized for their antimalarial properties. These structures are remarkable in that the indole nucleus is no longer present, having been rearranged into a quinoline system.
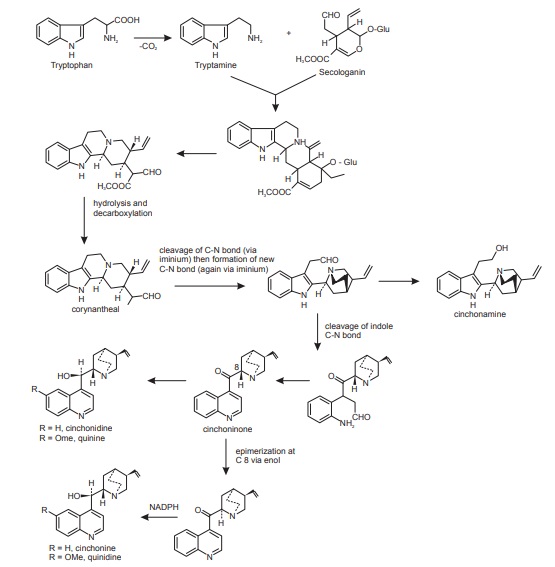
Biosynthesis of chinchona alkaloids
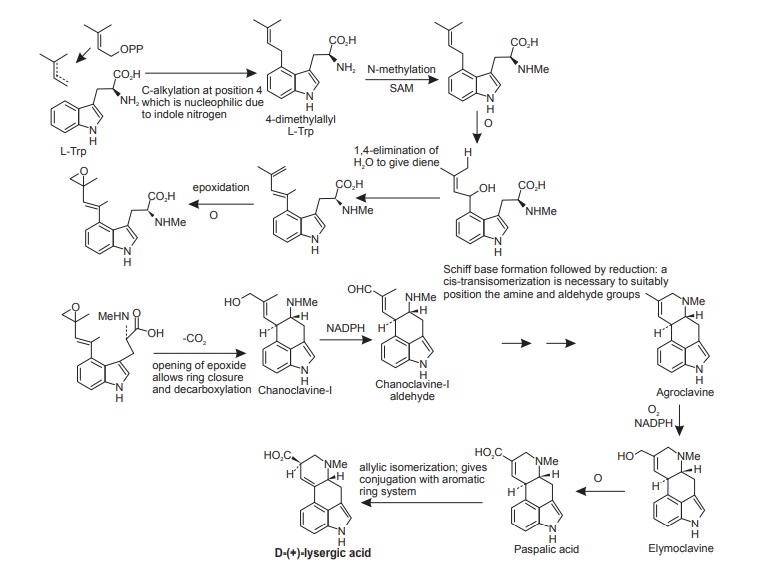
Biosynthesis of Lysergic Acid
The building blocks for lysergic acid are tryptophan (less the carboxyl group) and an isoprene unit (See the Figure below). Alkylation of tryptophan with dimethylallyl diphosphate gives 4-dimethylallyl-L-tryptophan, which then undergoes N-methylation. Formation of the tetracyclic ring system of lysergic acid is known to proceed through chanoclavine-I and agroclavine, though the mechanistic details are far from clear. Labelling studies have established that the double bond in the dimethylallyl substituent must become a single bond on two separate occasions, allowing rotation to occur as new rings are established. This gives the appearance of cis–trans isomerizations as 4-dimethylallyl-L-tryptophan is transformed into chanoclavine-I, and as chanoclavine-I aldehyde cyclizes to agroclavine. A suggested sequence to account for the first of these is shown. In the later stages, agroclavine is hydroxylated to elymoclavine, further oxidation of the primary alcohol occurs giving paspalic acid, and lysergic acid then results from a spontaneous allylic isomerization.
Related Topics
