Statistical Methods of Evaluating Pharmacovigilance Data
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Statistical Methods of Evaluating Pharmacovigilance Data
The three main challenges of pharmacovigilance, that is to detect, to assess and to prevent risks associated with medicines (Bégaud, 2000), may concern both the patient level and the populational level.
Statistical Methods
of Evaluating Pharmacovigilance Data
INTRODUCTION
The
three main challenges of pharmacovigilance, that is to detect, to assess and to
prevent risks associated with medicines (Bégaud, 2000), may concern both the
patient level and the populational level. Similarly, the latter may rely on
classical epidemiological studies, for example cohort or case-control, or on
cases-only anal-yses, which is the scope of spontaneous reporting (SR).
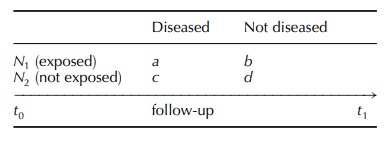
In cohort studies (Kramer, 1988),
subjects are followed in a forward direction from exposure to outcome (e.g. the
occurrence of a given disease), and inferential reasoning is from cause to
effect. For exam-ple, in the case of a cohort study with a reference group, the
subjects can be split, at the end of the follow-up, among the four cells of the
following clas-sical two-by-two table:
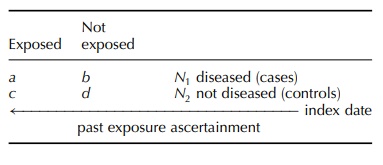
In case–control studies, subjects
are investigated in a backward direction, from outcome (disease) to expo-sure
and inference is from effect to cause:
In
both designs, the compared groups are generally drawn from a larger source
population, which raises the problem of possible selection biases; however, the
subjects are generally exhaustively classified according to a binary variable:
to present or not to present the considered disease in cohort studies, or to
have been or not to have been exposed to the studied factor in case–control
studies. SR, per se, is a passive
surveillance method involving the whole source-population, for example all
subjects of as given country treated with a given medicine; however, SR suffers
two major limitations (Bégaud, 2000):
·
it does not provide any direct and reliable infor-mation on
the size, characteristics and exposure patterns of the source population;
·
the term spontaneous
refers to the random charac-ter of the case collection from the exposed
popu-lation; indeed, reporting assumes that the observer
(i) identifies the adverse event,
(ii) imputes its occurrence to a drug exposure, (iii) is aware of the existence
of a pharmacovigilance system, and (iv) is convinced of the need to report the
case if relevant, for example new and/or serious adverse drug reactions (ADRs).
This
results in the major plague of this surveil-lance method: an inescapable under-reporting, the magnitude and
selectivity of which are unknown and extremely difficult to assess. Indeed, if
a number a of cases of a given event have occurred in a population during the
‘follow-up’ period, then it is likely that only a part k = a/U of these cases will be
reported, U being the under-reporting
coefficient varying from 1 to infinity, for example U = 4 if 25% of cases have been
reported.
Moreover,
it is hard to believe that each of the a cases that have occurred have an
identical probability 1/U to be reported. Many factors have been shown to
influence reporting (Pierfitte et al.,
1999) such as the age of the patient, the seriousness of the event and its
onset delay. Thus, because of a selection bias, k could be a non-representative
sample of the source population of cases.
From
a biostatistical point of view, the rather bizzare design of SR could be
compared to a cohort study without reference group in which:
·
the ‘followed’ population is extremely large, that is the
whole population of the surveyed territory treated with drugs;
·
the characteristics of this population, for example age and
gender distributions, concomitant diseases, are unknown as are its
characteristics of exposure (indications, dose, duration, co-medications,
etc.);
·
the number of ‘investigators’ is extremely large, that is
all health professionals in the territory;
·
the case collection does not rely on a precise protocol and
is thus non-systematic and may be subjective.
Moreover, because of the open character
of this method of surveillance (any type of drug, any type of event), there is
in fact a quasi-infinite number of sub-cohorts, one for each type of drug
exposure:
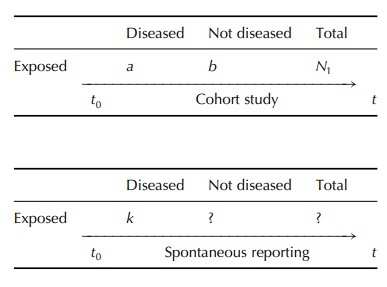
While
the cohort study can estimate the risk associated with a given drug exposure by
calculation of the inci-dence rate a/N1t (number of new occurrences
of the disease produced by the surveyed population during the period t), to
estimate risks from SRs requires rather complex assumptions and calculations.
Related Topics
