The Netherlands Pharmacovigilance Centre Lareb
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Pharmacovigilance in the Netherlands
Spontaneous Reporting in the Netherlands: The Netherlands Pharmacovigilance Centre Lareb
SPONTANEOUS REPORTING IN THE NETHERLANDS: THE NETHERLANDS PHARMACOVIGILANCE CENTRE LAREB
INTRODUCTION
The
organization of the reporting of adverse drug reactions in the Netherlands can
be characterized as follows:
• a strong involvement of reporters (physicians, pharmacists and patients);
• a strong relationship with the scientific world, resulting in a scientific way of working and the development of new approaches for the processing and the analysis of the data;
• a principal choice for transparency: Lareb want to be accountable for the reports it received and the results of its analyses.
In all these Lareb’s renewed website (www.lareb.nl) plays a vital role.
DIRECT RESPONSIBILITY OF DOCTORS AND PHARMACISTS
Lareb
is an organization which was founded by doctors and pharmacists and which is
still the responsibility of doctors and pharmacists. All large medical and
pharmacists’ associations and patient organizations are represented on its
administrative board. Lareb maintains the national ‘spontaneous’ reporting
system for the Netherlands. That this task falls to an independent centre
rather than the govern-ment sets the Netherlands apart from most other coun-tries.
Although some (such as Germany, New Zealand and Great Britain with its Drug
Safety Research Unit) have organizations investigating adverse drug reac-tions
that are allied to universities or professional organizations, the involvement
of professional prac-titioners is particularly prominent in the Netherlands.
The government restricts itself to a supervisory and coordinating role, while
it also provides funding for Lareb’s activities.
The
Dutch model has a significant number of advan-tages and works very well in
practice. It is doctors and pharmacists who encounter adverse drug reactions in
day-to-day practice. Given co-responsibility for the proper monitoring of drug
safety, they will be more inclined to contribute. This enhances the premise
that doctors and pharmacists are themselves responsible for the safe and
responsible use of prescription drugs. The barriers to reporting suspected
adverse reactions will be significantly lowered if those reports are made to a
peer group organization. After all, the occurrence of an adverse reaction may
cause the doctor or phar-macist to ask himself (or herself) whether he should
assume partial responsibility for this reaction. It is possible that some would
be less eager to report an adverse drug reaction to a ‘higher authority’ such
as the government. Reporting adverse drug reactions is voluntary in the
Netherlands.
REPORTING BY PATIENTS
Since
April 2003 patients are allowed to report expe-rienced adverse drug reactions
to Lareb. They may do so through an adjusted web form at Lareb’s website. The
first year (1 April 2003 till 1 April 2004) Lareb received a total of 276
reports from patients. The second year, 726 patient reports were registered
(van Grootheest, Passier and van Puijenbroek, 2005).
In
general, the reports are of a good quality. It is the patient who uses the
drugs and experiences any adverse drug reactions personally. Therefore, it is
obvious that patients are involved in the prevention of adverse drug reactions
and are willing to report their experiences.
In
the past, involvement of patients played an important role in drawing attention
to the adverse reactions of DES, benzodiazepines and antidepres-sants. Another
consideration is the fact that more and more drugs are available without a
doctor’s prescrip-tion. To obtain information on adverse reactions of ‘over the
counter’ drugs, patients’ reports may play an important role.
The
important question: do patients’ reports add to the reports of doctors and
pharmacists is not unanimously answered in literature. The fact that this
question cannot be answered without prior practi-cal experience was an
important argument for Lareb to decide to accept patients’ reports (van
Grootheest et al., 2004a).
Both
in patients’ reports and reports from health professionals, the part of reports
concerning females is higher than that concerning male patients. The mean age
of the female patients is comparable in patients’ and health professionals’
reports; the mean age of male patients is a bit lower in patients’ reports. Lareb’s
decision to let patients report only via the website may have put a
disadvantage to, for example, the elderly.
Analysing
the severity of the adverse drug reac-tions, 29% of the patients’ reports
appears to be ‘seri-ous’ versus only 21% of the adverse drug reaction reported
directly by health professionals. A report is considered ‘serious’ when the
adverse drug reaction led to hospitalization, death of the patient, a
congen-ital anomaly or persisting disability, according to the CIOMS criteria.
Arranged
into system organ classes, the five most reported adverse drug reactions
received from patients as well as doctors and pharmacists show a remarkable
similarity. In both groups disorders of the nervous system are most frequently
reported, whereby dizzi-ness and fatigue were often reported by patients.
When
the reports are classified into drug classes, according to the ATC system,
patients as well as health professionals report most often on psychotropic
drugs. Patients appear to report mainly on adverse reactions of
antidepressants. The second most frequently reported drugs by patients are sex
hormones, a drug class not in the top ten of the health professionals’ reports.
On
the basis of these positive findings in the first year, Lareb decided to
continue accepting patients’ reports. Reporting patients receive a personal
reac-tion with comments on the content of the reported adverse drug reaction.
When analysing data from Lareb’s database, patients’ reports are seen as full
reports. However, the source of a report is always mentioned, so this can be
considered during analyses. The reliability of the reporting system as a whole
has improved, since patients’ reports are taken seriously.
REGIONAL ORGANIZATION
The
Netherlands Pharmacovigilance Centre Lareb, in which several professions meet,
has an extensive network of doctors and pharmacists. This is indeed facilitated
by the Lareb’s regional organization under which the Netherlands is divided
into five regions. The Lareb’s headquarters in ‘s-Hertogenbosch acts as one
regional office, with the other four in university hospitals throughout the
country. Each regional office has a regional coordinator, responsible for
maintain-ing contact with the doctors and pharmacists in that region.
Furthermore,
the regional coordinator personally assesses some of the incoming reports in
order to remain involved in the Lareb’s ‘core business’ and will contribute to
relevant publications wherever possible. A meeting of all Lareb’s scientific
staff is held monthly at the headquarters, providing an oppor-tunity for
consultation and further ‘in-service’ train-ing. Lareb is a small organization,
with a staff of only 19. Some work part-time. There are five supportive
(administrative) staff members, the remainder are all doctors, pharmacists or
medical biologists by profes-sion. Details can be found on Lareb’s website at
www.lareb.nl.
MARKED INVOLVEMENT OF PHARMACISTS
In
the context of pharmaceutical patient care, phar-macists in the Netherlands are
highly involved in ensuring the safe and responsible use of medicines.
Pharmacists played an important part in setting up the Lareb Centre. Today,
pharmacists (see Figure 22.1) provide about 40% of the reports the Centre
receives.
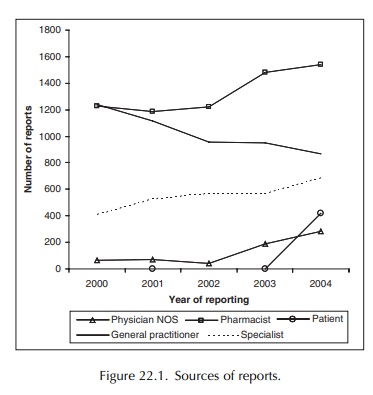
Most reports are made by community pharma-cists, which perhaps can be expected given the Lareb’s background. Hospital pharmacists lay some-what behind in this respect. Accordingly, Lareb has joined forces with the Netherlands Society of Hospi-tal Pharmacists in attempting to encourage greater involvement on the part of its members. One of the objectives is to establish a protocol in hospitals whereby house pharmacists are not only expected to provide effective pharmacotherapy, but will also play a coordinating and facilitating role in terms of the collation and forwarding of adverse drug reaction reports. A survey held in early 2001 indicated that 97% of hospital pharmacists are eager to report any adverse reactions; they know what must be reported and in what way. In practice, the complaint that phar-macists provide little or no clinical information in a report has not appeared much of a problem. Often, the good cooperation between doctors and pharmacists ensures adequate information to be given, particularly if the relevant report is made in a hospital situation. If necessary, it is possible to contact the prescrib-ing doctor to obtain further information. The fact that pharmacists are able to provide a complete picture of a patient’s prescription history is a significant advantage.
THE GENERATION OF SIGNALS
The
primary objective of any reporting system is to generate a ‘signal’: an early
indicator or warning of a potential problem. This may be compared to the task
of a fire-watcher, who looks for smoke and, if he thinks he spots it, must then
determine whether there is indeed a fire and where that fire is located.
Sometimes additional research is needed. In pharmacovigilance, it falls to the
Medicines Evaluation Board to determine whether there are sufficient arguments
to shout ‘fire!’, whereupon it will take the necessary measures.
Computer
automation plays an important role in the internal report assessment process,
with all incoming reports undergoing a set sequence of events. The infor-mation
on the report forms themselves, together with that in any other relevant
documentation, is stored in digital form next to archiving the paper copies of
the reports. At the time of writing (early 2005) Lareb’s database contained
over 50,000 reports.
Reports
received by Lareb are first assessed by one of its staff doctors or
pharmacists. They exam-ine the probability of a causal link, and will use the
current literature, previous reports and the description of the drug’s
pharmacological mechanism to assist them. The results of their assessment are
notified to the reporter as well as to the government. Next to the initial
assessment, feedback-information is provided to the reporter. Furthermore, the
assessors also focus on the possibility of the existence of possible signals in
this stage. In addition, serious reports are also assessed by one of the senior
staff members, who also pays special attention to the possibility of the
existence of a signal.
The
reports and their subsequent assessments are discussed in a weekly assessment
meeting. The aim of this meeting is primarily the detection of possi-ble
signals. It is determined whether or not further action is necessary. Such
further action may entail more detailed analysis of the relationship between
the reported reaction and the suspect drug or follow-up information to be
provided by the reporter. Research within Lareb has revealed a number of
factors that can play a significant role in the decision to conduct further
analysis. These include the seriousness of the reported reaction, whether or
not the association has been reported disproportionally, the presence of a
so-called WHO critical term and whether or not the reported ADR is labelled in
the Summary of Product Characteristics (van Puijenbroek et al., 2001b).
The
weekly assessment meeting also makes use of information obtained through
automated quantita-tive signal generation and external sources of infor-mation.
A Reporting Odds Ratio is calculated for all reports, providing a statistical
indication of the reporting frequency of each of the suspected reactions
compared with other reports in Lareb’s database (van Puijenbroek et al., 2002; van Puijenbroek, Diemont
and van Grootheest, 2003). The results of the Bayesian Confidence Propagation
Neural Network analysis, submitted quarterly by the World Health Organization
Monitoring Centre in Uppsala, are also automatically linked to each report.
Based on the information on suspected and concomitant drug the existence of
possible pharmacodynamic or pharmacokinetic drug–drug interactions is
automatically highlighted by the computer system. Also the possible
involve-ment of the cytochrome system in drug–drug inter-actions or the
possibility of a genetic polymorphism of the cytochrome system in the
pathogenesis of adverse drug reactions is automatically monitored for. Finally
prescription-data provided by the Dutch Health Care Insurance Board (CVZ) are
linked to the reports, providing information about the number of prescriptions
and the number of ADR-reports per 100,000 prescriptions. The latter information
enables the assessors to identify possible unexpected increases in the number
of reports which may be indicative of the existence of a signal. Besides
providing a valu-able aid to case-by-case analysis, quantitative infor-mation
can also be used to distil useful information from a large collection of data.
Such information will not be provided by a single case analysis. Lareb is
particularly interested in the possibilities for identify-ing specific
syndromes and in detecting interactions
between drugs (van Puijenbroek et al.,
1999, 2000). Ongoing research is being conducted on whether certain risk
factors for drug reactions can be identified using the information filed in the
database.
After
assessment the reports are filed in the Lareb-database. An anonymized copy of
the reports fulfilling the definition of a ‘serious’ report according to the
CIOMS criteria is forwarded to the Marketing Authorization Holder of the
product in the Netherlands. In addition a copy of all reports is forwarded to
the WHO collaborating centre in Sweden (the Uppsala Monitoring Centre). Since
April 2005 serious reports are also forwarded to the European Medicines
Evaluation Agency to be filed in the Eudravigilance database.
The
increasing number of reports asks for the devel-opment of methods that enable a
triage of reports with and without a high signal value. In this triage process,
results from disproportionality analysis will be combined with more clinical
and pharmacological-oriented information. It is to be expected that the
development of this system will be completed by the end of 2005.
TRANSPARENCY
Lareb
has made a principal choice for maxi-mal transparency. In this decision,
Lareb’s website (www.lareb.nl) has a central place. At this website, all reports
received by Lareb can be examined per system organ class and per drug class. By
clicking on the indi-vidual case reports, more detailed information is
avail-able: demographic characteristics, information about (concomitant)
medication, reported adverse drug reac-tions and the outcome of the reactions
is provided. All reports and publications written by Lareb on received adverse
drug reactions can be viewed. Also, one can find standardized information on
frequently occurring adverse drug reactions. Naturally, reporting through the
website is possible; for health care
professionals and patients
different web forms are available. The main part of the website is translated
in English, since it may be interesting for other countries to view the reports
and other information owned by Lareb.
Doctors and Pharmacists
Because
Lareb itself is an organization of doctors and pharmacists, it has easy access
to practitioners in the field. Partly in view of the fact that doctors and
phar-macists report suspected adverse drug reactions on a purely voluntary
basis, it is important to inform and remind them of the importance of
reporting. In addi-tion to the feedback it provides, both direct and in the
form of publications, Lareb offers targeted informa-tion to potential reporters
in the form of mailings and presentations. The report form itself has a
carefully designed layout and is distributed in various ways, such as regular
inclusion with the Drug Bulletin and
the annual Farmacotherapeutisch Kompas,
the phar-macopoeia which forms a standard desk reference book for 90% of Dutch
doctors. An increasing number of reports is received in an electronic format
via the Lareb website.
It
is important that the reporter can rely on respect to privacy and
confidentiality. Lareb does not receive any information about the identity of
the patient and no information about the reporter will be given to third
parties. The Dutch law is strict on privacy.
An
important means of communication with the reporting parties is the ‘feedback
report’. Receipt of each report is acknowledged. Furthermore, the assess-ment
made by Lareb and the conclusions drawn with regard to the reported adverse
drug reaction are noti-fied to the reporter. Lareb strongly believes that the
feedback provided stimulates additional reporting in the nearby future. Once a
health professional submits an initial report to the Netherlands
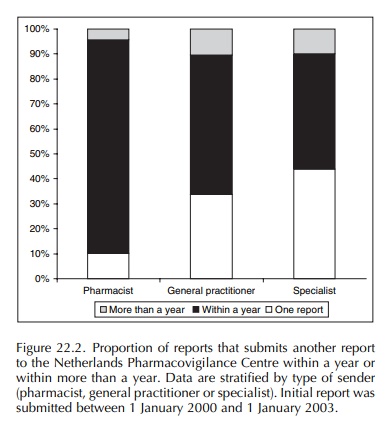
Pharmacovigilance Centre, the chance that he will report again within a year is
relatively high (Figure 22.2). A study analysing the chance for reporters to
submit another report in the nearby future shows that especially pharmacists
tend to report again within a year after the initial report has been received.
Besides wishing to encourage reporting, Lareb believes that it is important to raise the level of awareness among doctors and pharmacists with regard to adverse drug reactions. This will not only lead to a better standard of reporting, but will serve to significantly reduce the harmful effects of prescription medicines as well. Doctors will prescribe more crit-ically and will be more inclined to consider adverse drug reactions as the cause of complaints at an earlier stage in their differential diagnosis. Consequently, they will be able to either discontinue use of the drug or to adapt the dosage to avoid both unnecessary costs and unnecessary impact in terms of patient health.
The Government
Because
Lareb is an independent organization work-ing on behalf of the government, good
communica-tion with that government is very important. Reports are forwarded to
the Medicines Evaluation Board Agency weekly. Every six weeks, a meeting is
held with Lareb, the Agency and the Health Inspectorate. Besides possible
‘signals’, these meetings also discuss international developments. Lareb
participate in the meetings of the Medicines Evaluation Board.
Given
European developments, a more intensive cooperation is foreseen for the near
future.
Marketing Authorization Holders
Needless
to say, Lareb maintains close contact with the pharmaceutical industry, which
also has a vested interest in effective pharmacovigilance. All serious
(‘15-day’) reports are forwarded to the relevant Marketing Authorization
Holder, as required by inter-national legislation. These reports are anonymous,
neither the patient nor the reporter can be traced. Similarly, all serious
reports made directly by the pharmaceutical industry to the government are
entered into Lareb’s database. If such is wanted by the Market-ing
Authorization Holder also less serious reports will be send to them. All
articles concerning a specific preparation are submitted for comment to the
relevant Marketing Authorization Holder prior to publication.
RESULTS
The
‘output’ of Lareb can be assessed by looking at both the quantity and quality
of incoming reports, aspects that owe much to the efforts of the Centre. Other
criteria include the number of publications for which the Centre has been
responsible and the number of notifications of possible signals it has made.
Reports: Quantity
The
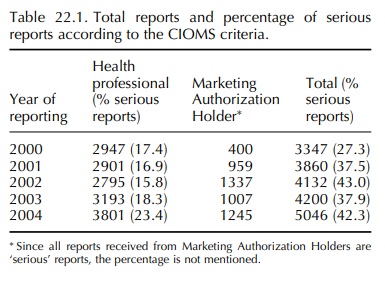
number of incoming reports continues to increase each year. The development in
the number of reports included in the database is shown in Table 22.1.
Reports: Quality
Although
an adequate number of reports is necessary to ensure a reliable reporting
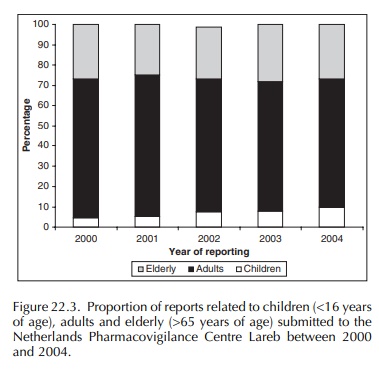
system, Lareb attaches greater importance to the quality of those reports. All age groups are represented in the
reports. Due to recent publications, the relative proportion of chil-dren (age
under 16 years) and elderly (age over 65) has risen in the past years (Figure
22.3). The steady increase in these age groups is especially important since
these patients appear to be the most vulnerable to adverse drug reactions.
The
quality of reports has also risen each year. Quality is continuously assessed
according to a number of criteria, one of which is the extent to which the
report is documented. In an increasing number of cases, reports are accompanied
by adequate clinical information, including the specialists’ clinical notes to
the patient’s family practitioner. The fact that more complete information is
now available may be partly attributed to the greater number of reports being
made by hospital practitioners.
Having adopted a scientific and academic level as the basis for its working methods, Lareb is regarded as a serious partner by other parties, particularly the professional organizations. The scientific quality of Lareb’s work is monitored by a Scientific Advisory Board, comprising experts in various disciplines. Each year, Lareb publishes over 30 articles in international or national journals, among which is the Dutch Drug Bulletin. Publications by Lareb can be downloaded from the website www.lareb.nl. Lareb also takes care of more than 30 presentations to groups of doctors and/or pharmacists and is frequently represented at international scientific conferences. Five theses have been published in relation to Lareb in the past years (De Koning, 1994; Egberts, 1997; Meyboom, 1998; van Puijenbroek, 2001a; van Grootheest, 2003a) and Lareb has contributed to several other theses and publications.
Related Topics
