Use of Microorganisms as Models of Mammalian Drug Metabolism
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : The Wider Contribution Of Microbiology To The Pharmaceutical Sciences
The safety and efficacy of a drug must be exhaustively evaluated before its approval for use in the treatment of human diseases. Investigations of the manner in which a drug is metabolized are extremely valuable as they potentially provide information on its mode of action, why it exhibits toxicity and how it is distributed, excreted and stored in the body.
USE OF MICROORGANISMS AS
MODELS OF MAMMALIAN DRUG
METABOLISM
The safety
and efficacy of a drug must be exhaustively
evaluated before its
approval for use
in the treatment of human diseases. Investigations of the manner in which a drug
is metabolized are extremely valuable
as they potentially provide information on its mode of action,
why it exhibits toxicity
and how it is distributed, excreted and stored in the body. Traditionally, drug metabolism studies have relied on the use of animal models
and, to a lesser
extent, liver microsomal preparations, tissue culture and perfused organ systems. Each
of these models
has certain advantages and disadvantages. Animals
in particular are expensive to purchase and maintain, and there is considerable pressure from
animal welfare groups
to curb the use of animals in scientific research.
The many similarities
between certain microbial enzyme systems and
mammalian liver enzyme
systems has led to the utilization of various microbial models for the exploration of mammalian drug
metabolism. The major advantages of using microorganisms are their ability
to produce
significant quantities of metabolites that would otherwise be difficult to obtain from animal systems
or by chemical synthesis, and the considerable reduction in operating costs compared with animal studies.
Microbial drug metabolism studies
are usually carried out by firstly
screening a large
number of microorganisms for their
ability to metabolize a drug substrate. The organism is usually
grown in a medium such as
peptone glucose
in flasks that are shaken
to ensure good aeration. Drugs as substrates are generally added
after 24 hours of growth
and are then sampled for the presence of metabolites at intervals up to 14 days after
substrate addition. Once it has been determined that a microorganism can metabolize a drug, the whole process
can be scaled up for the production of large quantities of metabolites for the determination of their structure
and biological properties.
As an example of this the metabolism of the antidepressant drug imipramine can be considered. In mammalian systems, this is metabolized to five
major metabolites:2-hydroxyimipramine,10-hydroxyimipramine, iminodibenzyl, imipramine-N-oxide and desipramine (Figure 26.10).
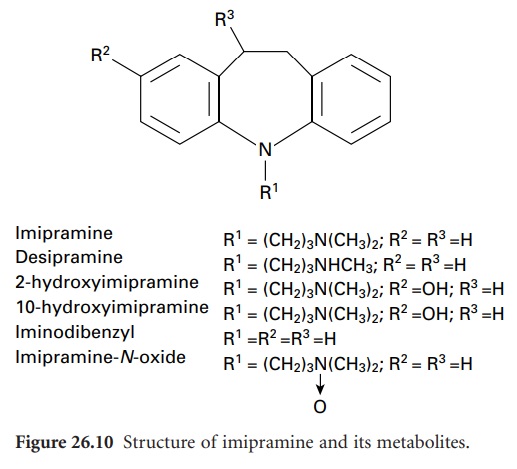
For microbial metabolism studies, a large number
of fungi are screened, from which several
are chosen for the
preparative scale production of imipramine metabolites. Cunninghamella blakesleeana produces the hydroxylated metabolites2-hydroxyimipramine and 10-hydroxyimipramine;
Aspergillus flavipes and Fusarium oxysporum
f. sp. cepae
yield the N-oxide
derivative and iminodibenzyl, respectively; while the pharmacologically active metabolite desipramine is produced
by Mucor griseocyanus together with the 10-hydroxy and N-oxide metabolites. By scaling up this procedure, significant quantities of the metabolites that are formed during mammalian metabolism can be obtained.
Microorganisms thus
have considerable potential as tools in the study
of drug metabolism. Although they cannot completely replace
animals, they are extremely
useful as predictive models for initial
studies.
Related Topics
