Antitubercular Agents : Standard drugs
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antitubercular Agents
i. Isoniazid (Continazin, Laniazid, Isonex, Ipcazide, INH, Isokin)) ii. Pyrazinamide iii. Ethambutol HCl (Myambutol) iv. Rifampicin v. Rifabutin vi. Streptomycin sulphate
Antitubercular Agents - Synthesis
and Drug Profile
Standard drugs
i. Isoniazid (Continazin, Laniazid, Isonex, Ipcazide, INH, Isokin))
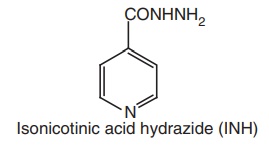
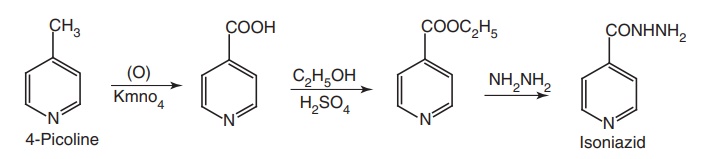
Synthesis
Mode of action: Isoniazid is a prodrug that is activated on the
surface of M. tuberculosis by katG
enzyme to isonicotinic acid. Isonicotinic acid inhibits the bacterial cell wall
mycolic acid, thereby making M. tuberculosis susceptible to reactive oxygen radicals. Isoniazid may be
bacteriostatic or bactericidal in action, depending on the concentration of the
drug attained at the site of infection and the susceptibility of the infecting
organism. The drug is active against susceptible bacteria only during bacterial
cell division.
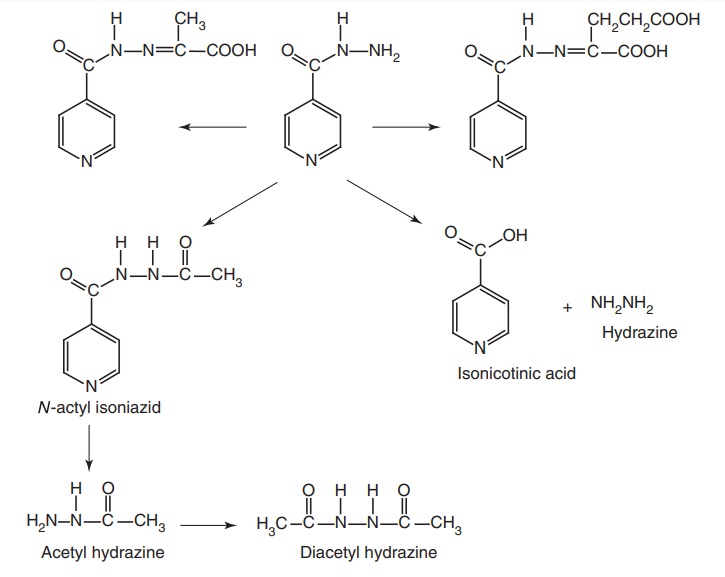
Metabolism: Isoniazid is extensively metabolized to inactive metabolites.
The major metabolite is N-acetyl
isoniazid. The enzyme responsible for acetylation is cytosolic N-acetyl transferase. Other metabolites
include isonicotinic acid, which is found in the urine as a glycine conjugate
and hydrazine. Isonicotinic acid also may result from hydrolysis of acetyl
isoniazid, but in this case, the second product of hydrolysis is acetyl
hydrazine. Acetyl hydrazine is acetylated by N-acetyl transferase to inactive diacetyl product. It has been
suggested that a hydroxylamine intermediate is formed that results in an active
acetylating agent.
Properties and uses: Isoniazid exists as white crystalline powder or
colourless crystals, soluble in water, and sparingly soluble in alcohol. It is
used as an antituberculosis drug.
Assay: Dissolve and dilute the sample with water, add hydrochloric
acid, potassium bromide, and methyl red and titrate drop wise with 0.0167 M
potassium bromate, shaking continuously, until the red colour disappears.
Dose: For the prophylaxis in case of adults is 5 mg/kg, with a maximum
of 300 mg. For children: 10–20 mg/ kg daily. Combination therapy Isoniazid,
Rifampin and Pyrazinamide for 2 months followed by Isoniazid (15 mg/kg orally)
with. Rifampin (10 mg/kg upto 600 mg per dose) twice/week for 4 months.
Dose: For the prophylaxis in case of adults 5 mg/kg, with a maximum of
300 mg.
Dosage forms: Isoniazid tablets I.P., B.P. Isoniazid injection B.P.
ii. Pyrazinamide
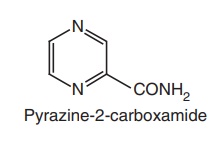
Synthesis
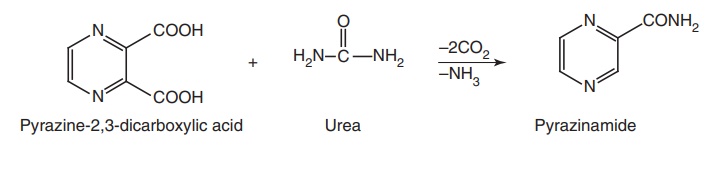
Route I. From: Pyrazine-2, 3-dicarboxylic acid
Route II. From: Pyrazine-2, 3-diamine
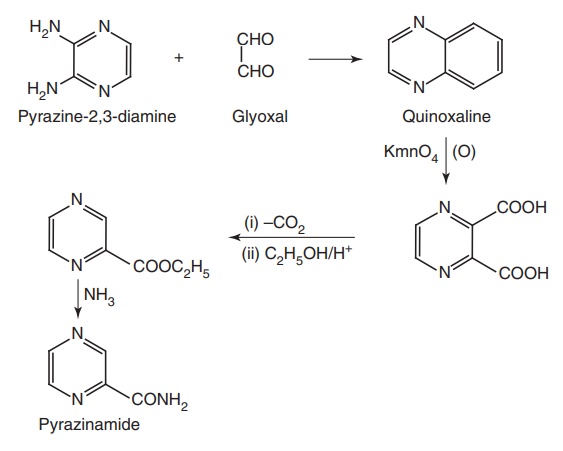
Metabolism: The metabolic route constitutes of hydrolysis by hepatic
microsomal pyrazinamidase into pyrazinoic acid, which may be then, oxidized by
xanthine oxidase to 5-hydroxy pyrazinoic acid. The later compound may appear
free either in the urine or as a conjugate with glycine.
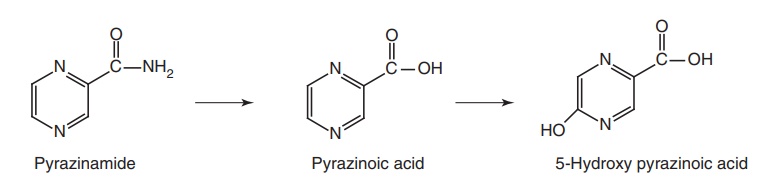
Properties and uses: Pyrazinamide is a white crystalline powder,
sparingly soluble in water, slightly soluble in alcohol and in methylene
chloride. It is a prodrug and is activated by M. tuberculosis amidase enzyme into pyrazine carboxylic acid, which
has bactericidal activity. Pyrazinamide has recently been elevated to first-line
status in the short-term treatment of tuberculosis regimens because of its
tuberculocidal activity and comparatively less short-term toxicity.
Pyrazinamide is maximally effective in the low pH environment that exists in macrophages
(monocytes). It is used to treat tuberculosis and meningitis. The drug should
be used with great caution in patients with hyperuricaemia or gout.
Assay: Dissolve the sample in acetic anhydride and titrate with 0.1 M
perchloric acid. Determine the end point potentiometrically.
Dose: Daily administered dose is 20–35 mg/kg in 3–4 equally spaced
doses and maximum is 3 g daily.
Dosage forms: Pyrazinamide tablets B.P.
iii. Ethambutol HCl (Myambutol)
Synthesis
Route I. From: 1,2-Dichloroethane
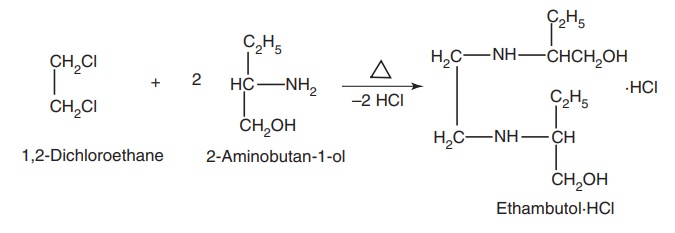
Route II. From: Nitropropane
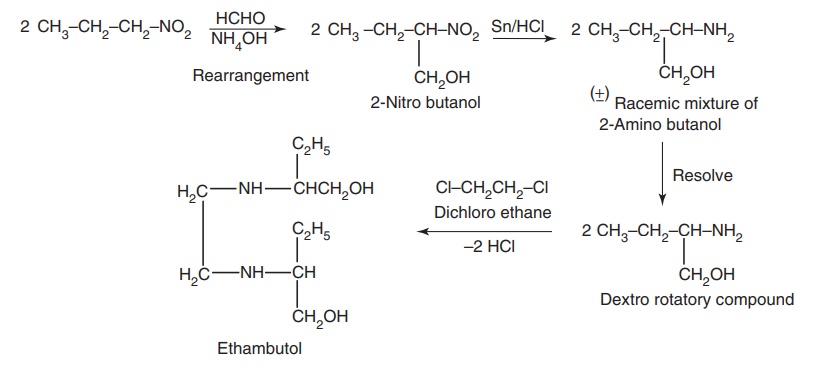
Metabolism: The majority of the administered ethambutol is excreted
unchanged (73%), with not more than 15% appearing in the urine as a metabolite,
which are devoid of biological activity.
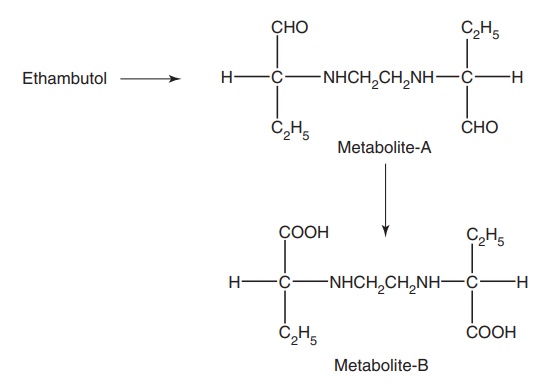
Mode of action: It is a bacteriostatic drug that inhibits the
incorporation of mycolic acid into the mycobacterium cell wall.
Properties and uses: Ethambutol hydrochloride is a white crystalline
powder, soluble in water and in alcohol. It is not recommended for use as a
single drug, but used in combinations with other antitubercular drugs in the
chemotherapy of pulmonary tuberculosis.
Assay: Dissolve and dilute the sample with solution of dilute ammonia,
copper sulphate solution, and dilute sodium hydroxide, and measure the angle of
optical rotation of the solution at 436 nm.
Dose: The administered dose is 15–25 mg/kg once a day; low doses for
new cases, and high doses for use in patients who have had previous antitubercular
therapy.
Dosage forms: Ethambutol HCl tablets I.P., Ethambutol tablets B.P.
iv. Rifampicin
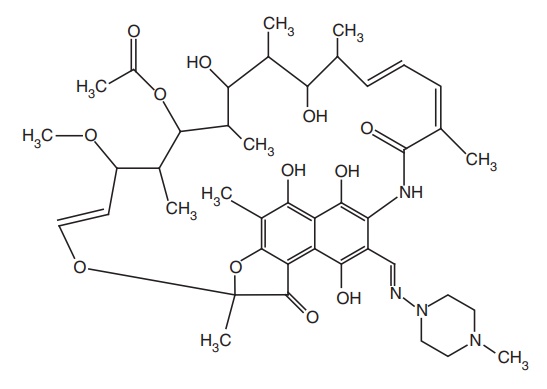
Mode of action: It is an antibiotic obtained from Streptomyces mediterranei. Rifampicin
inhibits DNA-dependent RNA polymerase of mycobacteria by forming a stable drug
enzyme complex, leading to suppression of initiation of chain formation in RNA
synthesis and acts as a bactericidal drug.
Metabolism: The major metabolism of rifampicin and rifapentine is deacetylation,
which occurs at the C-25 acetate. The resulting products, desacetyl rifampin,
and desacetyl rifampentine are still active antibacterial agents.
3-Formylrifamycin has been reported as a second metabolite following both
rifampicin and rifampentine administration.
Properties and uses: Rifampicin is a reddish-brown or brownish-red
crystalline powder, slightly soluble in water, acetone, alcohol, and soluble in
methanol. Rifampicin is the most active agent in clinical use for the treatment
of tuberculosis. It is used only in combination with other antitubercular
drugs, and it is ordinarily not recommended for the treatment of other
bacterial infections when alternative antibacterial agents are available.
Assay: Dissolve and dilute the sample in methanol. Dilute the solution
with phosphate buffer solution pH 7.4 and measure the absorbance at the maxima of 475 nm,
using phosphate buffer solution pH 7.4 as blank.
Dosage forms: Rifampicin tablets I.P, Rifampicin capsules I.P, B.P.,
Rifampicin oral suspension B.P.
v. Rifabutin
It is a semisynthetic
rifamycin, structurally similar to rifampicin. It is used against M. avium, one of the most common causes
of disseminated infections, with patients suffering with Human Immunodeficiency
Virus (HIV). In vitro activity is attributed to rifabutin’s lipophilic nature
and its ability to penetrate the cell wall of the organism more effectively
than other agents.
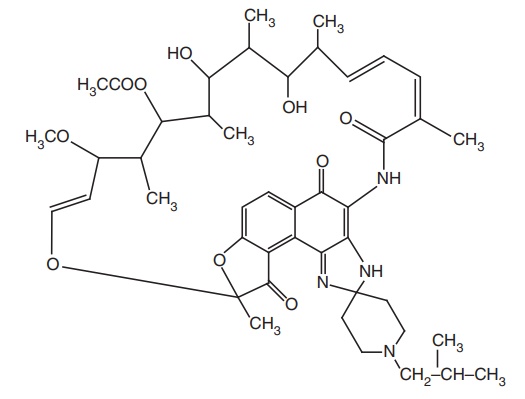
Properties and uses: Rifabutin is a reddish-violet amorphous powder,
slightly soluble in water and alcohol, soluble in methanol. It is used as an
antitubercular drug.
Assay: It is assayed by adopting liquid chromatography technique.
vi. Streptomycin sulphate
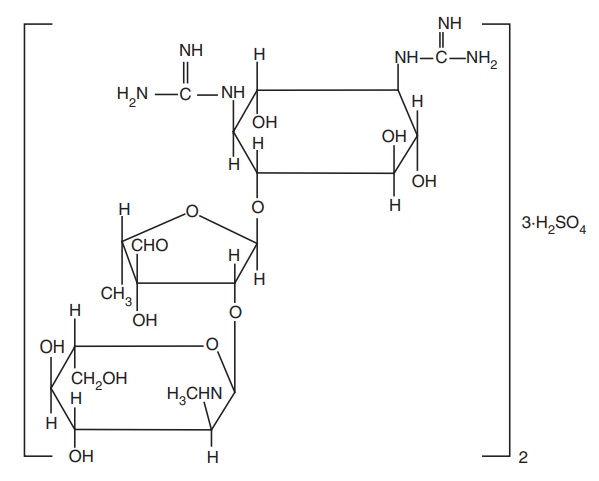
Metabolism: The enzymes responsible for inactivation are adenyltransferase,
which catalyzes adenylation of the C-3 hydroxyl group in the N-methyl glucosamine moiety to give the O-3-adenylated metabolite and
phosphotransferase, which phosphorylates the same C-3 hydroxyl to give O-3 phosphorylated metabolite.
Properties and uses: Streptomycin is a white hygroscopic powder, very
soluble in water, and practically insoluble in ethanol. It was the first
effective drug for the treatment of tuberculosis. It is most often used in
combination with other drugs, such as ethambutol and isoniazid, to treat pulmonary
infections in patients with organisms that are known to be resistant. There has
been an increasing tendency to reserve streptomycin products for the treatment
of tuberculosis.
Assay: It is assayed by adopting microbiological assay method.
Dosage forms: Streptomycin sulphate injection I.P., Streptomycin sulphate
tablets I.P., Streptomycin injection B.P.
