Macrolide antibiotics
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antibiotics
The macrolide antibacterial agents are extremely useful chemotherapeutic agents for the treatment of a variety of infectious disorders and diseases caused by a host of gram-positive bacteria, both cocci and bacilli;
Macrolide antibiotics
The
macrolide antibacterial agents are extremely useful chemotherapeutic agents for
the treatment of a variety of infectious disorders and diseases caused by a
host of gram-positive bacteria, both cocci
and bacilli; they also exhibit
useful effectiveness against gram-negative cocci,
specially, neisseria spp. The
macrolides are commonly administered for respiratory, skin, tissue, and
genitourinary infections caused by these pathogens.
Chemistry: They are characterized by five common chemical features.
1.
A
macrocyclic lactone usually has 12–17 atoms, hence the name macrolide.
2.
A ketone
group.
3.
One or two
amino sugars glycosidically linked to the nucleus.
4.
A neutral
sugar linked either to amine sugar or to nucleus.
5.
The presence
of dimethyl amino moiety on the sugar residue, which explains the basicity of
these compounds, and consequently the formation of salts. The antibacterial
spectrum of activity of the more potent macrolides resembles that of penicillin.
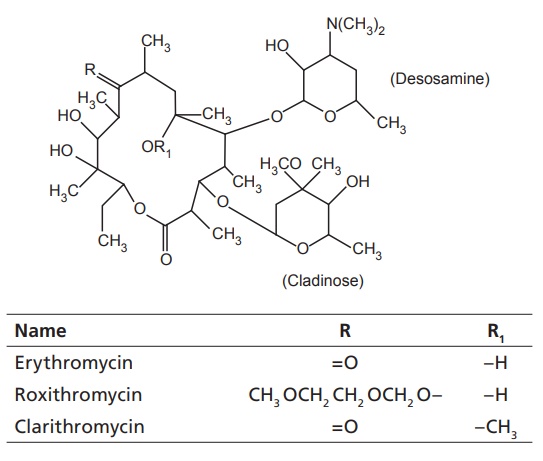
Examples: erythromycin, oleandomycin, clarithromycin, flurithromycin,
dirithomycin, azithromycin.
i. Azithromycin
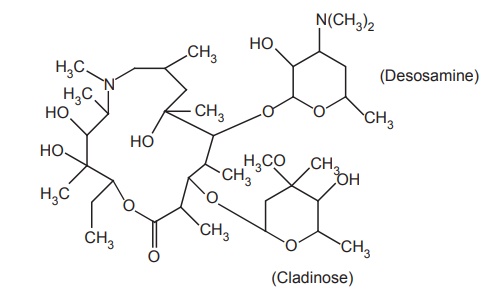
Properties and uses: Azithromycin is a white powder, practically
insoluble in water, soluble in anhydrous ethanol and methylene chloride. It is
very stable under acidic conditions, is less active against Streptococci and Staphylococci than erythromycin, and is far more active against
respiratory infections due to H.
influenzae and Chlamydia trachomatis.
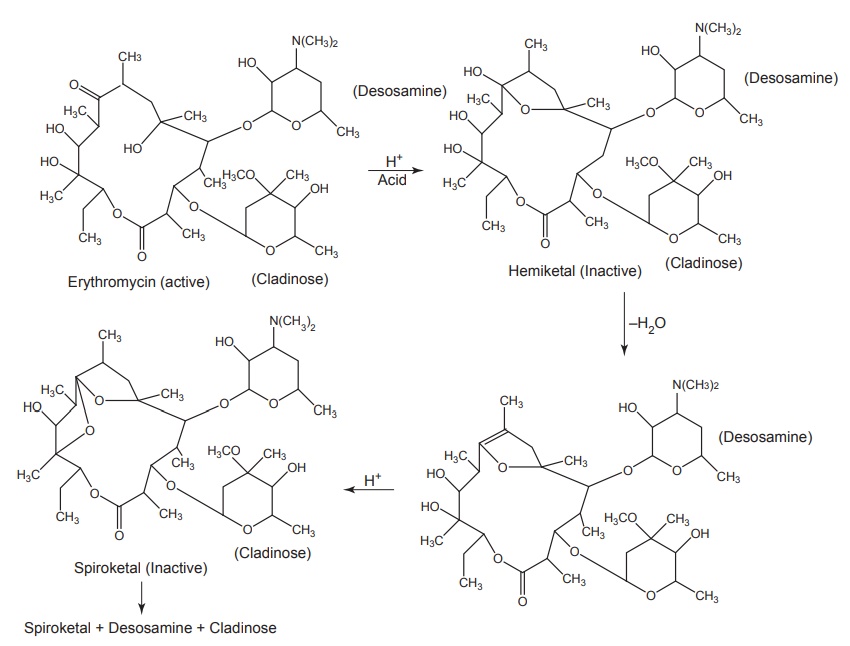
Acid degradation of erythromycin
Erythromycin
is unstable in the acid media. The C-6 hydroxyl group reversibly attacks the
C-9 ketone giving rise to a hemiketal intermediate. Dehydration prevents
regeneration of the parent erythromycin and the C-12 hydroxyl group can
subsequently add to produce a spiroketal species. The cladinose group is
cleaved from the macrocycle and more harsh conditions lead to the release of
desosamine. Useful antibacterial activity last till the dehydration of the
hemiketal and the spiroketal is weakly active.
Mode of action: Macrolide antibiotics are bacteriostatic agents
that inhibit protein synthesis by binding irreversibly to a site on the 50S
subunits of the bacterial ribosome. Thus, inhibiting the translocation steps of
protein synthesis at varying stages of peptide chain elongation (hinder the
translocation of elongated peptide chain back from ‘A’ site to ‘P’ site). The
macrolides inhibit ribosomal peptidyl transferase activity. Some macrolides
also inhibit the translocation of the ribosome along with the mRNA template.
Related Topics
