Stability
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Dosage forms - Emulsions
Emulsions must demonstrate physical, chemical, and microbial stability throughout their shelf life under recommended packaging and storage conditions.
Stability
Emulsions
must demonstrate physical, chemical, and microbial stability throughout their
shelf life under recommended packaging and storage conditions.
Physical instability
Physical
stability of an emulsion is characterized by the maintenance of elegance with
respect to appearance, odor, color, taste, opacity, and viscos-ity. Four major
phenomena are associated with the physical instability of emulsions: (1)
flocculation, (2) creaming, (3) coalescence, and (4) breaking. These phenomena
are schematically illustrated in Figure 17.2.
Flocculation is discussed under the chapter on suspensions.
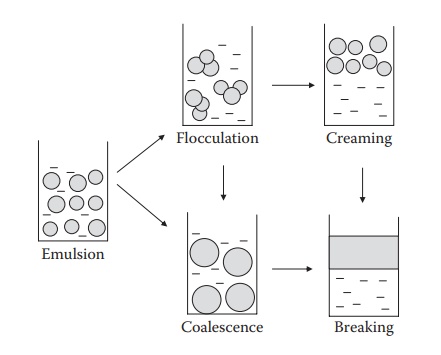
Figure 17.2 Schematic illustrations of different types of instability of emulsions.
Creaming and sedimentation
Creaming is the upward
movement of dispersed oil droplets in an o/w
emulsion, whereas sedimentation,
the reverse process, is the downward movement of dispersed-phase droplets.
Creaming involves visually evident separation of two layers that differ
primarily in the number density of the dispersed phase, and, thus, show optical
differences. These processes take place due to the density differences in the
two phases and can be reversed by shaking. Creaming is undesirable because a
creamed emulsion increases the likelihood of coalescence due to the closer
proximity of the globules in the cream and because of the nonuniformity of the
creamed emulsion. The propensity and rate of creaming is influenced by factors
similar to those involved in the sedimentation of suspensions and are indicated
by the Stoke’s Law, as discussed in a preceding Section
17.4.3.
Aggregation, coalescence, creaming, and breaking
Aggregation
involves close packaging/contact of the dispersed-phase drop-lets, but the
droplets do not fuse. Aggregation is, to some extent, reversible. Coalescence
is the process by which emulsified globules merge with each other to form
larger globules. Coalescence is an irreversible process because the film that
surrounds the individual globules is destroyed. It leads to pro-gressive
increase in the size of the dispersed phase, ultimately leading to breaking of
the emulsion. Breaking of an emulsion refers to complete sepa-ration of the two
liquid phases. Creaming is a reversible process, whereas breaking is
irreversible. When breaking occurs, simple mixing fails to resus-pend the
globules in a stable emulsified form, since the film surrounding the particles
has been destroyed and the oil tends to coalesce. The proportion of the volume
of emulsion occupied by creamed layer is an indicative of the stability of an
emulsion. Greater the proportion of the creamed layer, more stable the
emulsion. Flocculation, which leads to weak interactions between
dispersed-phase droplets, can stabilize an emulsion by increasing the dura-tion
of time it takes for creaming and the proportion of the creamed phase.
Formation
of a thick interfacial film is essential to minimize coalescence. In addition,
increasing the mechanical strength of the interfacial barrier, such as by
closer packing of the interfacial surfactant monolayer, reduces the propensity
toward coalescence. Increasing the viscosity of the continu-ous phase helps to
stabilize the dispersed phase and minimizes coalescence.
Particle
size does not correlate well with increased/decreased breaking, nor does
viscosity. Phase volume is an important consideration in the stability of an
emulsion. For example, at greater than ~74% of oil in an o/w emulsion, the oil
globules often coalesce and breaking occurs. Thus, a critical concentra-tion is
defined in terms of the concentration of the internal phase above which the
emulsifying agent cannot produce a stable emulsion of the desired type.
Generally, a phase-volume ratio of 50:50 results in the most stable emulsion.
Phase inversion
An
emulsion is said to invert when it changes from an o/w to a w/o emul-sion, or
vice versa. Phase inversion can occur by the addition of an elec-trolyte or by
changing the phase volume ratio. Addition of monovalent cations promotes the
formation of o/w emulsions, whereas the addition of divalent cations increases
the propensity toward the formation of w/o emulsions. For example, an o/w
emulsion stabilized with sodium stearate can be inverted to a w/o emulsion by
adding calcium chloride to form cal-cium stearate.
Chemical instability
The
API must be chemically stable in the dosage form throughout the shelf life of
the product under recommended packaging and storage conditions in terms of both
potency and impurities. The drug product must meet pre-determined requirements
of minimum potency of the API and maximum levels of known and unknown
impurities. Factors governing the reaction kinetics of the API, such as the
reactivity of functional groups and the kinetics of reactions are no different
for emulsion dosage forms than other solution-based dosage forms. Nevertheless,
separation of the reacting spe-cies in the oily and aqueous phases can minimize
reactivity and improve stability of a drug in an emulsion.
Microbial growth
Microbial
load of a dosage form must be controlled within the compendial and the
regulatory levels. In addition to the health risks of microbial growth,
microorganisms in an emulsion can cause physical separation of the phases.
Preservatives must be added in adequate concentrations in the formulations to
resist microbial growth. The preservative should be concentrated in the
aqueous phase because bacterial growth will normally occur there. The oil and
water partition coefficient of the preservatives should be considered to
calculate the concentration of the surfactant in the aqueous phase, which needs
to be above the antimicrobial concentration. The para-bens (methylparaben,
propylparaben, and butylparaben) are the commonly used preservatives in
emulsions.
