Amphoteric Compounds
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Acidity and Basicity
A glance at the pKa values in Table 3.1 reveals that many classes of compounds can act either as acids or as bases, depending on the reaction environment. Such materials are termed amphoteric.
AMPHOTERIC COMPOUNDS
A
glance at the pKa values
in Table 3.1 reveals that many classes of compounds can act either as acids or
as bases, depending on the reaction environment. Such materials are termed amphoteric. They must have an acidic
proton (i.e., a proton attached to an electronegative element or group) and
unshared pairs of electrons that can be donated to a proton. For example,
water, alcohols, and other hydroxylic compounds as well as amines and amides
are all amphoteric materials. Comparing the pKa’s of these materials permits an assessment of the
predominant behavior in a given environment. For example, if an amine is
dissolved in water, it could function as an acid or a base. To determine which
behavior will predominate, the position of the equilibrium can be determined
for each process. Comparison of these values will indicate which will be the
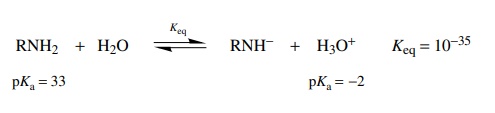
principal behavior. Thus, as an acid, the amine would donate a proton to water
to give an amide anion and the hydronium ion.
As
a base the amine would accept a proton from water to give an ammonium ion and
hydroxide.
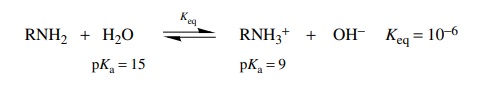
Since
the equilibrium constant for the amine acting as an acid is 10−35 and that for the
amine acting as a base is much larger at 10−6, reaction as a base
will be the main behavior in aqueous solution. The magnitude of the equilibrium
constant (10−6) indicates that it
is only a weak base.
On
the other hand, if you treat an amine such as diisopropyl amine with n-butyl lithium in tetrahydrofuran
(THF), then a different behavior is indicated. In this case the equilibrium
lies far to the right so as to be virtually irreversible. The amine therefore
functions as an acid and donates a proton to the much stronger base butyl
lithium. This is the standard method for the preparation of the versatile base
LDA.
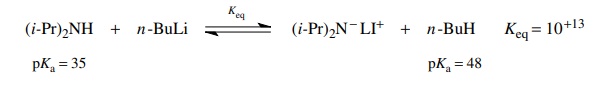
Related Topics
