Bronsted and Lewis Acids and Bases
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Acidity and Basicity
The notion of acids and bases is one of the first and most important ideas we are exposed to in chemistry, but it is one of the things that is often poorly understood.
BRONSTED AND LEWIS ACIDS AND
BASES
The
notion of acids and bases is one of the first and most important ideas we are
exposed to in chemistry, but it is one of the things that is often poorly
understood. The concept is actually very simple if a few basic ideas are always kept in mind. The first things
that must be remembered are the definitions of acids and bases and what general
structural features make compounds react as acids or bases.
Bronsted
acids are defined as proton donors. The dissociation of a Bronsted acid yields
a proton and the conjugate base (an
anion, if the acid is a neutral compound) of the acid.
HA
→ A− + H+
Bronsted
bases are proton acceptors. The only way that a Bronsted base can “accept” a
proton is to supply an electron pair and form a bond to the proton. Thus
Bronsted bases often are compounds with unshared pairs of electrons that can be
donated to a proton to form a bond. Upon reaction with a proton, a base is
converted to its conjugate acid.
B:
+H+ → B–H+
So
that charge is conserved, anionic bases upon reaction with a proton give
neutral conjugate acids; neutral bases upon reaction with a proton give
positively charged conjugate acids. Occasionally shared pairs of electrons can
be given up to a proton such as when olefins react with acids. In such cases π electrons are the electron pair which
forms a bond to the proton.
The
above two equations describing the behavior of Bronsted acids and bases are not
strictly correct because a Bronsted acid does not just dissociate, it donates a
proton to something which accepts a
proton. The proton does not just dissociate and float around in solution but is
always attached to something. Furthermore a Bronsted base does not just find a
proton to accept; it accepts a proton from a Bronsted acid. Thus acidity and
basicity are paired behavior—“you can’t have one without the other.” This is
the most common misconception about acids and bases and leads to the greatest
amount of difficulties when trying to apply the principles of acidity and
basicity to real reactions.
A
second description of acidity and basicity is the Lewis definition. A Lewis
acid is an electron acceptor and a Lewis base is an electron donor. The
definition of Lewis acids includes the proton since the proton can accept
electron pairs. Thus Bronsted acids are a subset of Lewis acids since all
Bronsted acids yield a proton. However, other common Lewis acids (BF3,
TiCl4, SnCl4, AlCl3) are routinely used as
organic reagents and all function by accepting unshared or shared electron
pairs. In general the electron pairs that are accepted are readily available;
that is, they are not tightly bound, so they are usually lone pairs or π electrons.
Lewis
bases are electron pair donors and the electrons are given up to Lewis acids
(electron acceptors). Unshared electron pairs are the more common type of
electron pairs to be donated to Lewis acids, although on occasion shared pairs
can be donated to the Lewis acid. In the case of Lewis acid–base reactions, the
product is termed a Lewis acid–base
complex.
Given these definitions, it is crucial to emphasize again that no compound is inherently an acid or a base. A compound only functions as an acid (in the Bronsted sense) if it donates a proton to something; that is, there must be a base present (a proton acceptor) to have an acid–base reaction. Thus a compound acts as an acid only in the presence of a base, and a substance can only act as a base in the presence of an acid. A good example of this concept is the fact that HCl in the vapor phase is an undissociated, covalent molecule. This is because there are no molecules present capable of accepting a proton from HCl. As a result, HCl does not function as an acid in the gas phase. If H2O is added, however, an immediate acid–base reaction takes place in which HCl donates a proton to water, which is capable of accepting a proton from HCl. In this reaction an unshared pair of electrons on the oxygen atom of water is donated to the proton and an O–H bond is formed. In its reaction with water, HCl can function as an acid only because water can function as a base to accept the proton.
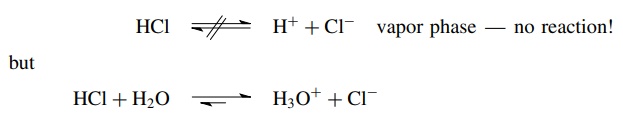
While
the necessity of having both and acid
and a base present in order to have an acid–base reaction is axiomatic, it is
surprising how often this concept is neglected. However, if these conditions
are met, then a wide variety of organic compounds can donate protons to
appropriate bases (they are deprotonated) and a wide variety of compounds can
accept protons from appropriate acids (they are protonated).
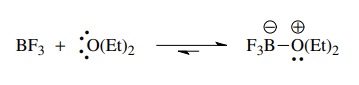
The
same considerations are true for Lewis acids and bases. Pure boron tri-fluoride
does not act as a Lewis acid because there is nothing present capable of
donating electrons to it. If diethyl ether is added, boron trifluoride
etherate, a stable Lewis acid–base complex, is produced. Obviously the electron
pairs on the oxygen atom of diethyl ether can be donated to boron trifluoride.
When a Lewis base is present, then boron trifluoride functions very effectively
as a Lewis acid.
Most
organic compounds do not act as Lewis acids because they are generally
closed-shell molecules with filled valence levels and no unfilled orbitals
capable of accepting electrons. By virtue of the unshared pairs of electrons on
oxygen and nitrogen atoms, organic compounds which contain these elements can
often function as Lewis bases toward many Lewis acids. Lewis acids such as BF3,
TiCl4, or SnCl4 are commonly used to react with
oxygen-containing Lewis bases such as carbonyl compounds, alcohols, or ethers.
Related Topics
