Oxidation States in Common Functional Groups
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Oxidation States of Organic Compounds
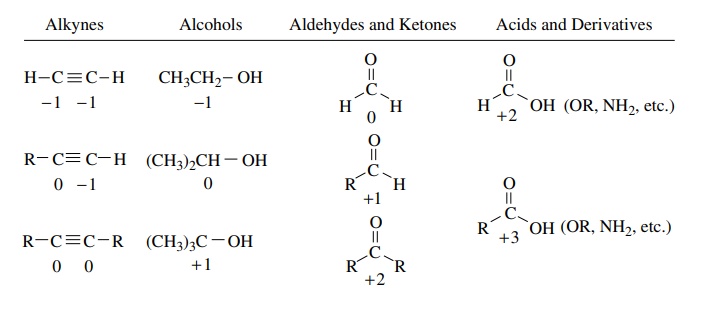
The same process can be carried out to determine the oxidation levels of car-bon atoms in several common functional types.
OXIDATION STATES IN COMMON
FUNCTIONAL GROUPS
The
same process can be carried out to determine the oxidation levels of car-bon
atoms in several common functional types. It is clear that by using these
procedures we can assign oxidation levels to carbon atoms in a wide variety of
compounds. It is also clear that knowing the oxidation level is insufficient to
assign the functional group present. For example, the alkane neopentane, the
alkene isobutylene, the alkyne propyne, the alcohol isopropanol, and
formalde-hyde all have a carbon with an oxidation level of 0 yet all belong to
completely different functional classes and have different physical and
chemical characteristics.
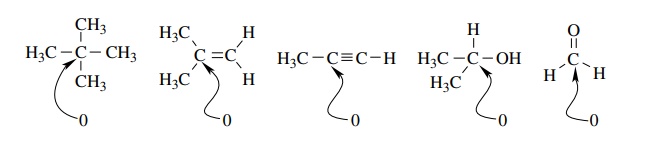
Thus
the oxidation level of a given carbon is dependent only on the groups which are
attached to it, not on the functional group to which it belongs.
Related Topics
