Oxidation States in Alkanes
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Oxidation States of Organic Compounds
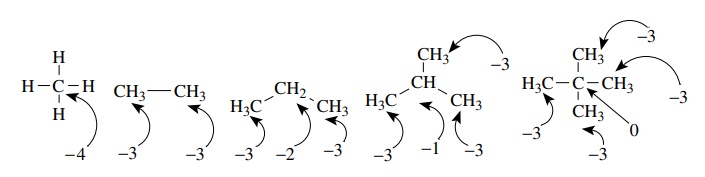
Considering the various alkanes shown below, one sees that alkanes can have several different oxidation levels for carbon.
OXIDATION STATES IN ALKANES
Considering
the various alkanes shown below, one sees that alkanes can have several
different oxidation levels for carbon. Oxidation levels can range from −4 for methane and −3 for the carbon atom of methyl
groups all the way to 0 for
the quaternary carbon of neopentane. In spite of the several oxidation levels
possible in alkanes, the functional group approach tells us that all are
saturated alkanes and thus have the same functional equivalency and similar
reactivity patterns.
This
conclusion is made from the fact that all the carbons in alkanes have four
single bonds originating from them and those σ bonds go to either carbon or hydrogen. Thus one cannot
automatically assign a molecule to a functional class based solely on a certain
oxidation level of the carbons that it contains.
Related Topics
