Conjugated Π Systems
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Groups and Chemical Bonding
Another way to describe delocalized bonding uses the MO approach. The same principles of overlap of AOs can be applied to systems where more than two p AOs overlap to form π systems.
CONJUGATED π SYSTEMS
Another
way to describe delocalized bonding uses the MO approach. The same principles
of overlap of AOs can be applied to systems where more than two p AOs overlap
to form π systems. First, the number
of MOs produced by the overlap will be the same as the number of atomic p
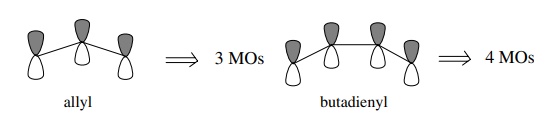
orbitals which interact. Thus for the allyl system where three contiguous p
orbitals interact, there will be three MOs produced from the interaction of
three 2p AOs. For the butadienyl system where there are four contiguous p orbitals
interacting, four MOs will result, and so on.
Second,
the energy distribution of the MOs will be disposed symmetrically about the
energy of the AOs before they interact (nonbonded energy level). This means
that the total energy of the bonding MOs is offset by the total energy of the
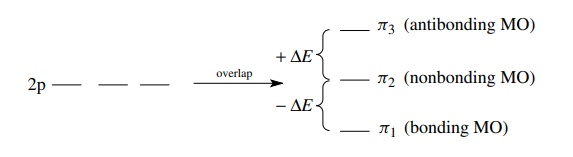
antibonding MOs. For example, if one MO is of lower energy by –ΔE due to
overlap, then there must be an antibonding MO raised to higher energy (+ΔE). Molecular orbitals which are
lower in energy than the nonbonding energy are bonding MOs, (−ΔE ), those which are higher in
energy than the nonbonding energy
are antibonding MOs (+ΔE),
and those at the same energy as the nonbonding energy are nonbonding MOs (ΔE = 0).
For
the allyl system which has 3 MOs from the overlap of three 2p AOs, one MO will
be lowered in energy (−ΔE
) and so one MO will be raised by the same amount. The remaining MO must stay
at the nonbonding level (ΔE = 0) to maintain energy symmetry around the
nonbonding level.
What
is interesting is that this overlap model allows the orbital diagram to be
constructed without concerning itself with electrons. The MOs produced by the
interaction of AOs can each hold two paired electrons, and these can be filled
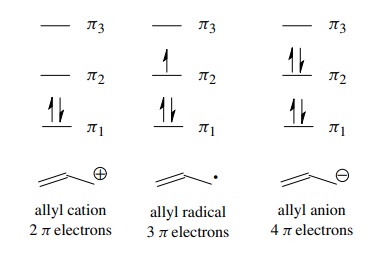
in depending on the number of electrons present in the π system. Thus the bonding diagrams for the allyl cation, allyl
radical, and allyl anion can be constructed by merely filling the orbitals with
the number of π electrons present in
these species (two, three, and four π
electrons, respectively). This orbital picture also demonstrates that all three
intermediates in the allyl system are stabilized because each contains two
electrons in the π1-bonding
MO and any remaining electrons are in the nonbonding orbital.
Two
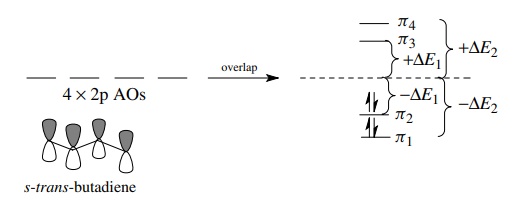
of the four MOs of the butadienyl system are at lower energy than the nonbonded
energy level (−ΔE1, − ΔE2), and two are at higher energy
than the nonbonded energy level (+ΔE1, + ΔE2). The four π
electrons of butadiene fill the two bonding MOs and give a stable molecule. It
should also be obvious that butadienyl species with less than or more than four
π electrons should be significantly
less stable than butadiene itself. Removal of an electron requires energy
because the electron would have to come from a relatively stable bonding MO.
Addition of an electron to the butadienyl π
system requires that it be put into an antibonding MO which is also
energetically unfavorable.
A
great many π systems have been
examined by this approach and the orbital diagrams understood. As seen before,
the antibonding orbitals are often unfilled in the ground state but play an
important part in the excited states and reactions of these compounds.
Related Topics
