Functional Groups
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Groups and Chemical Bonding
There are over 12 million known compounds of which more than 80% are organic compounds. To make sense out of the nearly 9 million organic compounds and be able to manipulate them and make new compounds, there must be some system of organization whereby organic compounds can be categorized by a particular property or group of properties.
FUNCTIONAL GROUPS
There
are over 12 million known compounds of which more than 80% are organic
compounds. To make sense out of the nearly 9 million organic compounds and be
able to manipulate them and make new compounds, there must be some system of
organization whereby organic compounds can be categorized by a particular
property or group of properties. A natural method utilized by early
practitioners was to group organic compounds by the reactions that they
underwent. Thus there developed a whole variety of qualitative tests called
classification tests which could be used to systematically categorize the
reactivity of a compound and thus allow it to be grouped with others of similar
chemical reactivity. These tests are still very useful to practicing organic
chemists and collectively are known as organic qualitative analysis.
Classification
tests are used to distinguish organic compounds and segregate them into
different functional classes based on their chemical properties. Orig-inally a
group of compounds that showed similar chemical behavior based on the
classification tests were named for a property or behavior (e.g., acids from acer meaning “sour,” aromatic compounds
from their odors). With the evolu-tion of the science of chemistry and the
development of more modern views of atoms and molecules, a different definition
of functional classes is possi-ble. The behavior of organic compounds is now
organized into patterns that are based on recurrent groups of atoms —
functional groups. The sites in molecules at which chemical reactions occur are
localized at the functional groups in the molecule; the rest of the molecule is
the same after the reaction as before. Thus, instead of thinking of the whole
molecule in terms of its chemical reac-tivity, it is only necessary to
recognize what functional group or groups are present in the molecule. It is
then possible to predict the chemical behavior of the molecule based on the
known chemistry of the functional groups that it contains.
This
turns out to be a huge simplification. Since the numbers of functional groups
are relatively small, it is possible to classify a very large number of
individual compounds by a relatively small number of functional groups. So the
first step to enlightenment in organic chemistry is to realize the key role
that functional groups play in simplifying the subject, and the second step is
to learn the functional groups by name, structure, and formula. While a great
number of them may have already been encountered in the introductory organic
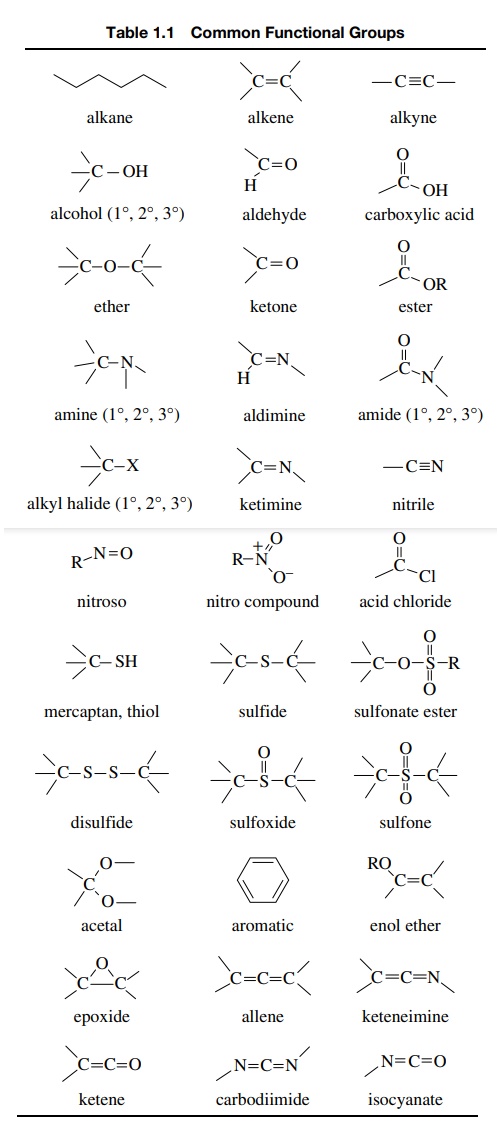
course, it is helpful to review them. Table 1.1 is a list of the most common
functional groups. While there are quite a few other functional groups that are
not shown, those found in Table 1.1 are the most common and are present in the
vast majority of organic compounds. Notice that not all functional groups
contain only carbon atoms (e.g., the nitro group and the carbodiimide groups),
and some functional groups differ at atoms other than carbon (compare the nitro
and nitroso groups and the sulfoxide and sulfone groups). Since functional
groups are reference points for predicting and understanding the reactions of
individual organic molecules, it is very important to be able to recognize
these functional groups (and others that might be encountered in the future).
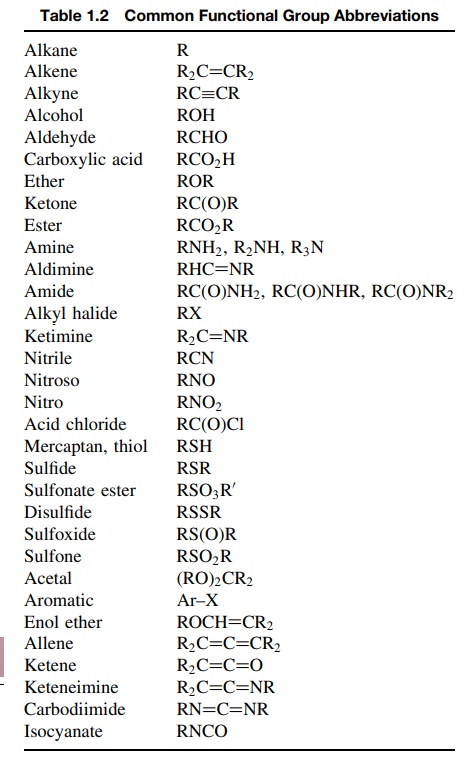
It is also useful to learn normal structural abbreviations that are used to
indicate functional groups that are present in chemical structures. The
abbreviations in Table 1.2 correspond to the groups that are shown in Table
1.1.
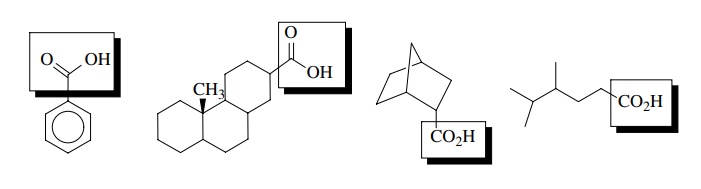
A major reason that the behavior of organic compounds can be generalized in terms of the functional groups they contain is because the bonds holding a given functional group together are the same regardless of the compound which contains that functional group. The four compounds shown below all contain the carboxylic acid functional group, which is highlighted within the boxes. Thus all four contain the bonding pattern characteristic of the –COOH functional group which is independent of the bonds found in rest of the molecule!
Since
most organic reactions involve the conversion of one functional group to
another, it follows that most organic reactions quite simply involve bond
changes involving functional groups. If one knows the bonds found in the
reactant functional group and the bonds found in the product functional group,
then one automatically knows what bonding changes are required to effect the
desired chemical change. Thus, in addition to being able to recognize
functional groups, it is also important to be able to describe the numbers and
types of bonds found in functional groups.
Bonds
in functional groups can first be described by Lewis structures, which are
merely formalisms for denoting numbers of shared and unshared electron pairs,
formal charges, and types of bonds (numbers of shared pairs, single, double,
and triple). Chemistry students learn to write Lewis structures in virtually
all of their early chemistry courses. How to write Lewis structures will not be
reviewed here, but knowing the correct Lewis structures for molecules and
functional groups in molecules is an indispensable first step in being able to
describe the structure and bonding of functional groups.
The next level of insight into functional groups comes from the translation of Lewis structures into more accurate bonding descriptions based on modern bonding theories. Structural details including geometries also result from the proper description of the bonding in the functional group. The ideas of structure and bonding currently in use had their origins in the late 1920s. It is again beyond the scope of this book to trace the developments which were seminal in the development of current theories; however, early studies were all rooted in the quest to understand and be able to describe the behavior of electrons in atoms. The development of quantum mechanics and the particle – wave duality of the electron and the uncertainty principle led to mathematical descriptions of the behavior of electrons in the electric field of the nucleus. The solution of those equations resulted in a new conceptual framework for understanding chemical bonding.
