What is chelation?
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Chelation Therapy
Chelation is the binding of molecules to a metal ion and is one of the most important concepts within the area of transition-metal complexes.
What
is chelation?
Chelation is the binding of molecules to a
metal ion and is one of the most important concepts within the area of
transition-metal complexes. The molecules or ligands in question are organic
compounds and can form more than one bond to the metal ion. These ligands are
classified as polydentate ligands, which means that this single ligand has more
than one atom that can bind to the central atom in a coordination complex. The
ligands are called chelators or chelating ligands (Figure 11.3).
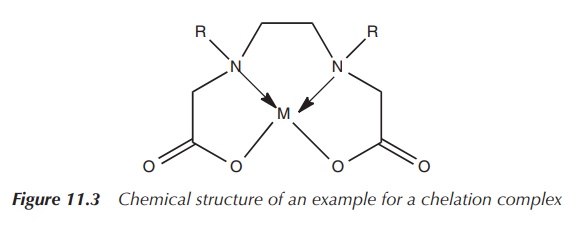
Chelation is defined as the formation of two or more coordinate bonds between a polydentate ligand and a single atom.
In terms of the nomenclature, the denticity of a ligand is
denoted by the Greek letter κ (kappa). If the denticity equals 1, it is a
monodentate ligand and only one bond from the ligand to the metal is formed.
For bidentate ligands, the denticity equals 2, which means that two coordination
bonds are formed from the ligand to the metal. One of the most known examples
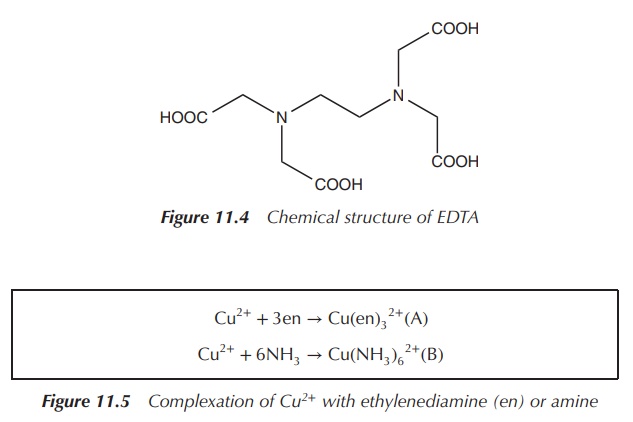
is EDTA (ethylenediaminetetraacetic acid), which is a hexadentate ligand. EDTA
can coordinate to a metal ion via six atoms and therefore is denoted as κ6-EDTA.
It is important not to confuse denticity with hapticity (see Chapter 8), which
only refers to bonds that are contiguous (Figure 11.4).
The term chelate effect
is used to describe the preferred binding of a chelating ligand to a metal ion
in comparison to the corresponding amount of monodentate (nonchelating) ligand
under the same reaction con-ditions. The most widely known example is the
reaction of a transition metal (e.g. Cu2+) with 1 equiv of the
bidentate ligand ethylenediamine (en) or with 2 equiv of the monodentate ligand
amine (NH3) (Figure 11.5).
Cu2+ + 3en → Cu(en)32+(A)
Cu2+ + 6NH3 → Cu(NH3)62+(B)
Figure 11.5 Complexation of Cu2+ with ethylenediamine (en) or amine
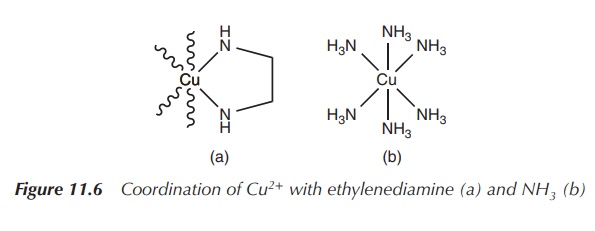
The reaction of the bidentate ligand ethylenediamine results in
the formation of a chelate complex in which the ligand forms two bonds to the
metal centre and a five-membered ring is formed (see Figure 11.6). The same reaction
takes places with 2 equiv of the monodentate ligand amine. Nevertheless, under
the same reaction conditions, the concentration of the five-membered
coordination complex (product A) will be significantly higher than product B.
This is called the chelate effect.
The chelate effect increases with the number of chelate rings formed. This explains why EDTA is a very good chelating agent, as it can form up to six coordinating bonds to the metal centre. In general, the chelate effect can be explained by using a thermodynamic approach, which considers the equilibrium constants for the reaction.
Nevertheless, this will
not be further discussed here, as it is beyond the scope of this book.
Related Topics
