Bonding Schemes
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Groups and Chemical Bonding
Bond formation between atoms occurs primarily to enable each atom to achieve an inert gas electron configuration in the valence level (a valence octet for all elements except hydrogen which requires only two electrons to achieve the electronic configuration of helium).
BONDING SCHEMES
Bond
formation between atoms occurs primarily to enable each atom to achieve an
inert gas electron configuration in the valence level (a valence octet for all
elements except hydrogen which requires only two electrons to achieve the
electronic configuration of helium). An atom can achieve an inert-gas
electronic configuration by giving up electrons, accepting electrons, or
sharing electrons with another atom. An ionic bond is formed when one atom
gives up one or more electrons to reach an octet electronic configuration (as a
positively charged ion) and a second atom accepts one or more electrons to
reach an octet electronic configuration (as a negatively charged ion). For
example, the reaction of a cesium atom with a fluorine atom occurs by the
transfer of an electron from the cesium atom to the chlorine atom. By doing so,
both cesium and chlorine have reached a valence octet electron configuration.
The cesium atom has been converted to a positively charged cesium ion with the
octet electronic configuration of xenon, and the chlorine has been converted to
a negatively charged chloride ion with the octet electronic configuration of
argon. The “bond” between cesium and chlorine is due to the electrostatic
attraction of the cesium and chloride ions.
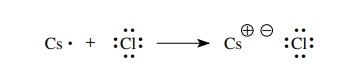
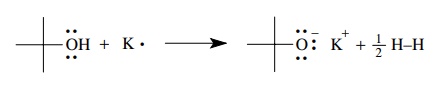
The
reaction of potassium metal with tert-butanol
gives an ionic bond between the tert-butoxy
anion and a potassium cation by transfer of electrons from potas-sium to the
hydroxyl functional group. Hydrogen is evolved as a by-product. By losing an
electron, potassium gains the octet electronic configuration of argon, oxygen
has an octet structure (three lone pairs and one pair of shared electrons), and
hydrogen has the electronic configuration of helium. (Based on functional group
behavior, any other alcohol is predicted to react with potassium in the same
way — and they do!)
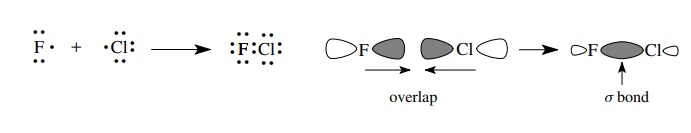
Most
bonds in organic molecules, however, are covalent bonds in which elec-trons are
shared between two atoms. Sharing electrons is a way to enable each atom of the
bonded pair to reach an octet electronic configuration without having to give
up or gain an electron. Covalent bonds are formed by the overlap of singly
occupied AOs to form new MOs that contain a pair of electrons. Each atom in
essence gains an electron by sharing. The reaction of a chlorine atom with a
fluorine atom occurs by the overlap of a singly occupied 3p orbital of chlorine
with a singly occupied 2p orbital of fluorine to give a bond between the two
atoms that contains two electrons. This is shown both by using Lewis structures
and by using orbital pictures. The type of bond formed is called a σ bond because the region of greatest
electron density falls on the internuclear axis.
This
simple picture is adequate for many diatomic molecules with univa-lent atoms,
but it is not sufficient to describe the bonding in most polyatomic molecules.
In addition to electron sharing to reach octet electronic configura-tions,
other considerations such as the number of bonds to an atom, the number of
electron pairs that are shared between two bonded atoms, and repulsion
ener-gies that are present between electron pairs require some modification of
the picture. These factors can be rationalized by the idea that valence shell
atomic orbitals (2s and 2p’s) can combine to form hybrid AOs. These hybrid AOs
over-lap with AOs of other atoms in the usual fashion to form covalent bonds.
Hybrid AOs have energies, shapes, and geometries which are intermediate between
the atomic orbitals from which they are formed. Hybridization of AOs is an
out-growth of bond formation that enables atoms to derive the greatest amount
of bond energy from electron sharing and to allow bonded atoms to achieve octet
electronic configurations.
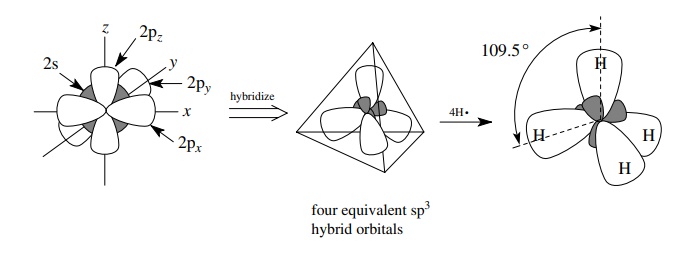
If
four single bonds and/or electron pairs originate from a single atom, then the
s orbital and the three p orbitals of the valence shell combine to form four
equivalent sp3 hybrid orbitals that are then used in bond formation
to other atoms. Depending on the number of electrons in the valence shell of
the atom, these sp3 hybrid orbitals can contain either a single
unpaired electron which can be shared with another atom by overlap and bond
formation or an unshared pair of electrons which is normally not involved in
bond formation. Thus alkanes, which have all single bonds, have carbon atoms
which are sp3 hybridized. For example, methane has four single C–H
bonds originating at carbon, and these bonds are σ bonds produced by the overlap of four sp3 hybrid
orbitals of carbon with four 1s AOs of four hydrogens to give four sp3
– 1s σ bonds from carbon to hydrogen.
The geometry of the four equivalent sp3 hybrid orbitals (and hence
the compound produced by overlap with these orbitals) is tetrahedral. Thus
methane has four equivalent C–H σ
bonds which point toward the corners of a regular tetrahedron and have H–C–H
bond angles of 109.5◦
:
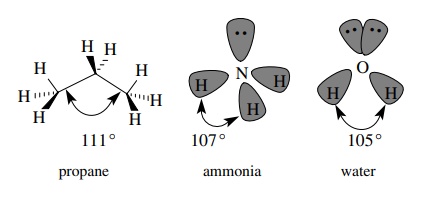
In
a similar fashion each carbon of propane is sp3 hybridized and
tetrahedral since each carbon has four single bonds to other atoms originating
from it. For example, the central carbon of propane has two equivalent sp3
– 1s C–H σ bonds and two equivalent
sp3 – sp3 C–C σ
bonds. (Note that sp3 orbitals from one carbon can overlap with sp3
orbitals from another carbon to produce carbon – carbon bonds.) The geometry is
very close to tetrahedral, but the C–C–C bond angle is slightly larger (111◦ ) to accommodate
the bigger CH3 groups.
Other
first-row elements can also be sp3 hybridized. The only requirement
is that they have a combination of four single bonds and/or electron pairs
originating from a single element. Ammonia, which has three N–H bonds and a
lone pair on nitrogen, is thus sp3 hybridized and has three
equivalent sp3 – 1s N–H σ
bonds and a lone pair which occupies an sp3 hybrid orbital. The
geometry is close to tetrahedral with an H–N–H bond angle of 107◦ . Other amines also
have sp3-hybridized nitrogen and are close to a tetrahedral geometry
around the nitrogen atom.
The
oxygen atom in the water molecule has two bonds and two lone pairs so it too is
sp3 hybridized. There are two equivalent sp3 – 1s O–H σ bonds and two lone pairs occupying sp3-hybridized
orbitals. Electron – electron repulsions of the lone pairs cause greater
distortions from a true tetrahedral geometry so that the H–O–H bond angle is
105◦ . Other singly
bonded oxygen functional groups such as alcohols, ethers, and acetals have sp3-hybridized
oxygens and nearly tetrahedral geometries.
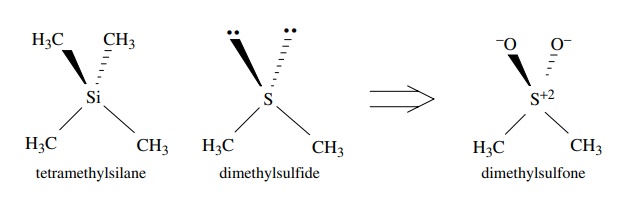
Second-row
elements such as silicon, phosphorus, and sulfur can also have sp3
hybridization of the valence shell orbitals, although hybridization is not
necessar-ily required for second-row elements. When second-row elements do
hybridize, however, the 3s and 3p AOs combine to form the sp3 hybrid
orbitals. Tetra-methylsilane, the standard reference for nuclear magnetic
resonance (NMR) spec-tra, has tetrahedral geometry and thus sp3
hybridization of the 3s and 3p valence shell orbitals of silicon. Dimethyl
sulfone has nearly tetrahedral bond angles, indi-cating that the sulfur is sp3
hybridized. Although formal charges are present, the two bonds to oxygen can be
thought to arise by the overlap of a filled sp3 orbital on sulfur
with an unfilled sp3 orbital on oxygen. The resulting σ bond is called a coordinate covalent,
or dative, bond because both of the shared electrons in the bond come from only
one of the bonded elements. Hydrogen sulfide has an H–S–H bond angle of 92◦ , which indicates
that sulfur is not hybridized in this compound.
When two pairs of electrons are shared between two elements, a different bonding arrangement is required to enable the atoms to reach valence octet elec-tron configurations. Because of the Pauli exclusion principle, only one sigma bond is possible between any two atoms because only one pair of electrons can occupy the space along the internuclear axis. The second pair of electrons that is shared by the two atoms must therefore be located in space someplace other than along the internuclear axis. The second pair of shared electrons is located in a different type of covalent bond, a π bond, which has electron density found on either side of the internuclear axis. The π bonding results from the parallel overlap (or sideways overlap) of atomic p orbitals. To accommodate the need for a singly occupied atomic p orbital available for the formation of a π bond, hybridization of the valence AOs takes place between the s orbital and two of the three p atomic orbitals. Hybridization of one s and two p AOs produces three equivalent sp2 hybrid AOs and a p orbital remains unhybridized in order to produce a π bond.
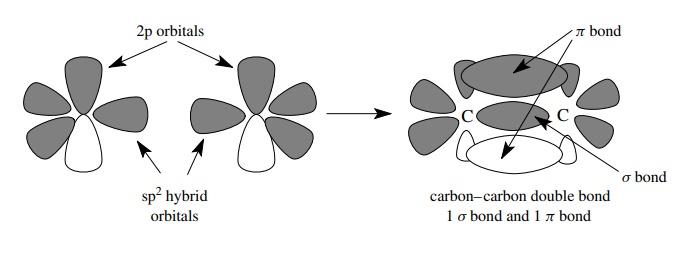
This
bonding scheme permits two pairs of electrons to be shared between two atoms so
that each pair occupies a different region of space and does not violate the
Pauli exclusion principle. Since only two p orbitals are used in the
hybridization and they are orthogonal and define a plane, the sp2-hybridized
carbon is planar with bond angles of 120◦ . The remaining p orbital, which is
left unhybridized to form the π bond,
is perpendicular to the molecular plane. Once formed, the π bond keeps the entire system rigid and planar, because rotation
of one end of the π -bonded system
relative to the other end requires that the π
bond be broken.
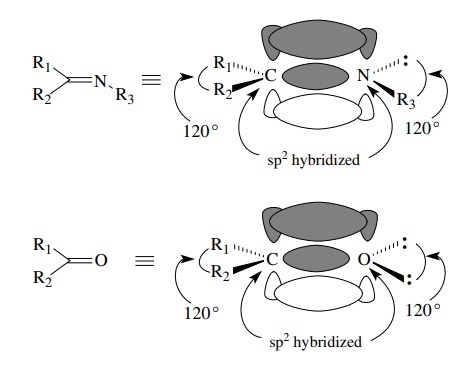
Elements
other than carbon are also sp2 hybridized if they share two electron
pairs with another atom. Thus imines have sp2-hybridized nitrogen
(and carbon) to account for formation of the C–N double bond. The lone pair on
nitrogen occupies an sp2 hybrid orbital. The bond angles are all 120◦ around both carbon
and nitrogen since both are sp2 hybridized. Similar considerations
hold for the oxygen atom of carbonyl groups of all kinds. The two unshared
pairs of electrons on oxygen both occupy sp2 orbitals. The
interorbital angle is 120◦
, as expected for trigonal hybridization.
The
sharing of three pairs of electrons between two atoms can be accomplished by
extrapolation of the above considerations. That is, since there can only be one
σ bond connecting the atoms, then the other two pairs of shared electrons must
be in two different π bonds, each of
which is formed by the parallel overlap of a p orbital. Furthermore the π bonds must be mutually orthogonal so
as not to violate the Pauli exclusion principle. Hybridization of one s orbital
and one p orbital gives two equivalent sp hybrid AOs which are linearly
opposite to one another.
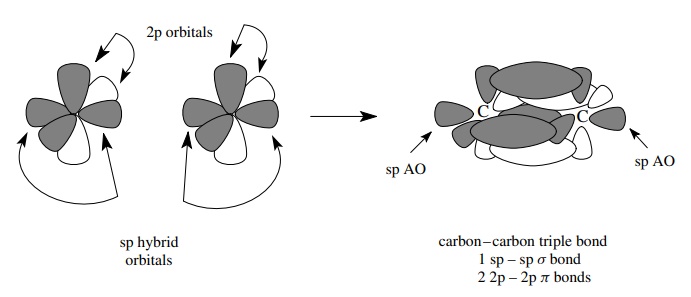
The
two remaining p atomic orbitals, which are mutually orthogonal, are used to
produce two orthogonal π bonds. The
geometry of triply bonded systems is thus linear about the triple bond.
Similar
considerations apply to the triply bonded nitrogen found in nitriles. The
sp-hybridized carbon and nitrogen atoms form an sp – sp σ bond and two 2p – 2p π
bonds between carbon and nitrogen. The unshared pair on nitrogen occupies an sp
hybrid orbital.
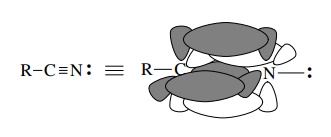
Another
instance where sp hybridization is required occurs in molecules with cumulated
double bonds such as allenes, ketenes, and carbodiimides. The end atoms of the
cumulated units are sp2 hybridized because each shares two electron
pairs with another element (the central carbon) and there is a σ and a π bond. The structure, however, requires that two π bonds originate from the central
carbon — one π bond going toward one
end of the cumulated system, the other π
bond going toward the other end. Thus two 2p AOs are required for π bonding from the central carbon and sp
hybridization is appropriate. Consequently the geometry is linear at the middle
atom and trigonal at the end atoms. A further consequence of the orthogonal π bonds is that planar bonds originating
at the end carbons lie in two orthogonal planes with a dihedral angle of 90◦ . (A dihedral angle
is the angle made by two intersecting planes.)
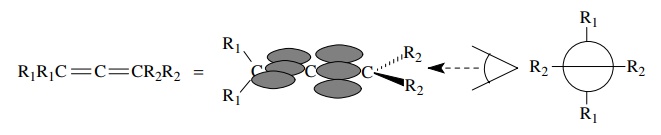
Besides
providing a theoretical framework by which the structure, geometry, and octet
structure of bonded elements can be explained and understood, the concept of
hybridization also predicts the ordering of stabilities and energies of bonds
and the energy of lone pairs of electrons in hybrid orbitals. Because s AOs are
of lower energy than p AOs, hybrid orbitals with a greater proportion of s
character should be more stable and thus form stronger bonds. Unshared pairs of
electrons in hybrid orbitals with greater s character should also be of lower
energy (more stable). As the percentage of s character of hybrid orbitals
increases in the order sp3 – 25% s character < sp2 – 33% s character < sp – 50% s character, it is found that the strength of bonds
formed by overlap with those orbitals increases in a parallel fashion. For
example, the bond dissociation ener-gies of primary C–H bonds have been
measured and fall in the order that is predicted by the percentage of s
character of the hybrid orbitals on carbon: sp3 C–H, 105 kcal/mol;
sp2 C–H, 111 kcal/mol; and sp C–H, 133 kcal/mol. Elec-tron pairs are
more stable in orbitals with more s character; thus the acidities of primary
C–H bonds are found to be sp3 C–H, pKa =
50; sp2 C–H, pKa
= 44; and sp C–H, pKa = 25. This is due to the fact that
the anions formed by pro-ton removal give carbanions that have the negative
charge in sp3, sp2, and sp orbitals, respectively.
Because the lone pair is more stable in an orbital of greater s character, the
anion formed by removal of an sp C–H proton is more sta-ble (and hence the
proton is more easily removed) than the anion formed by removal of an sp2
C–H proton, which in turn is more stable (and hence the proton is more easily
removed) than the anion formed by removal of an sp3 C–H proton.
Other examples of the effects of greater s character in orbitals are
encountered routinely.
The
concept of hybridization of AOs to give new hybrid AOs involved in the bonding
patterns of atoms is a useful and practical way to describe the way in which
functional groups are constructed. It provides a rationale for the structure as
well as the geometry and electron distribution in functional groups and
molecules in which they are found. It can also be used to predict reactivity
patterns of functional groups based on these considerations.
