Radiopharmaceuticals for imaging
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Radioactive Compounds and Their Clinical Application
Radiopharmaceuticals are typically administered intravenously and then distributed to a particular organ. The molecule itself, and not the radiolabelling, will determine to which organ the radioactive molecule is transported.
Radiopharmaceuticals
for imaging
Radiopharmaceuticals are typically administered intravenously and then distributed to a particular organ. The molecule itself, and not the radiolabelling, will determine to which organ the radioactive molecule is transported. γ-Radiation is detected externally by using a special scintillation detector, also known as a gamma camera. The camera captures the emitted radiation and forms a two-dimensional image. This diagnostic test is also called scintigraphy.
In contrast, PET produces a three-dimensional image of the
functional processes in the human body. The method is based on the use of
positron-emitting radionuclides and their indirectly emitted gamma rays.
Radionuclides, the so-called tracers, are introduced to the body as parts of
biologically active molecules. PET also uses gamma cameras to detect the
internally applied radiation, but in modern scanners, three-dimensional images
are often achieved with the aid of a CT X-ray scan performed at the same time
as part of the same machine.
Diagnostic X-ray uses external radiation, which is sent through the body to produce a two-dimensional image, whereas scintography is based on the internal accumulation of radionuclides.
1. 99mTechnetium
Technetium has the chemical symbol Tc and atomic number 43. It
is the lightest element that has no stable isotope. It is a silvery-grey
transition metal (Figure 10.17).
99mTc (also referred to
as technetium-99m) is the metastable
isomer of 99Tc, which is a gamma-emitting nuclide routinely used in
diagnostic medicine. It has a short half-life of around 6 h, which is ideal for
diagnostic applications (but not for therapeutic applications) as it helps to
keep the radiation exposure to the patient low. The use of a gamma camera
allows detection of the radioactive tracer in the body and creates images of
the area in question (Figure 10.18).
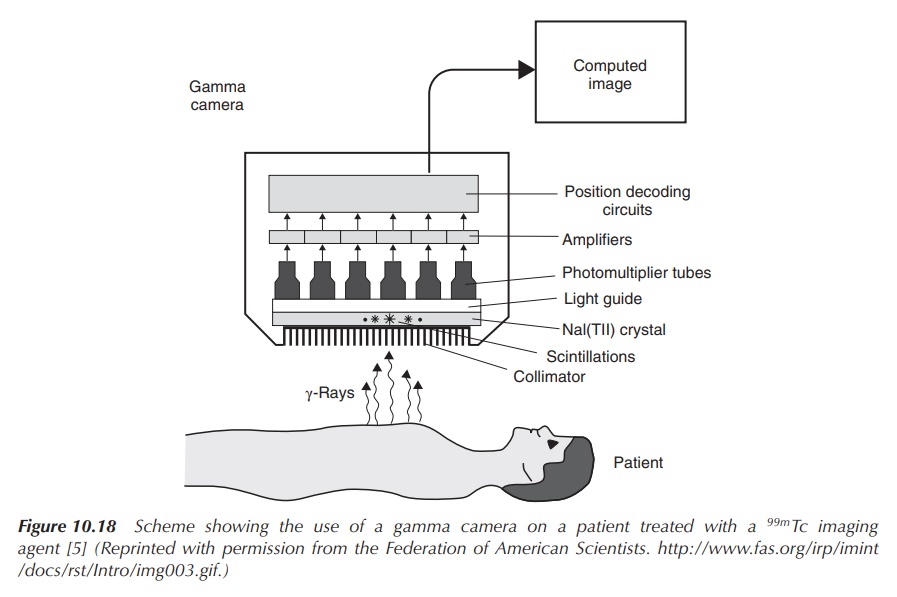
Figure 10.18 Scheme showing the use of a gamma camera on a patient treated with a 99mTc imaging agent (Reprinted with permission from the Federation of American Scientists . )
One challenge of using a radioactive material is to safely manufacture the products and deliver them to the clinical setting. Radionuclides with long half-lives are usually prepared commercially using a nuclear reactor and supplied as the finished product. Products containing radionuclides with a short half-life cannot be delivered as the finished product because of their rapid decay. Therefore, they are delivered to the clinical setting as radionuclides with a long half-life and the desired radionuclide is then generated and formulated at the moment of use. 99mTc and its compounds are generated in situ for use as an imaging agent using a so-called 99mTc generator. The generator is loaded with molybdenum-99 (99Mo), which is often referred to as the commercially available transportable source of 99mTc. The general idea is that the generator contains a long-lasting ‘parent’ compound, which decays and produces the ‘daughter’ radionuclide. In the case of the 99mTc generator, it contains 99mMoO42− absorbed on an alumina column. 99mMoO42− decays to 99mTcO4−, which can be removed as Na99mTcO4 when the column is washed with a NaCl solution. Hospitals tend to buy these generators on a regular basis to provide a continuous supply of 99mTcO4− (Figure 10.19).
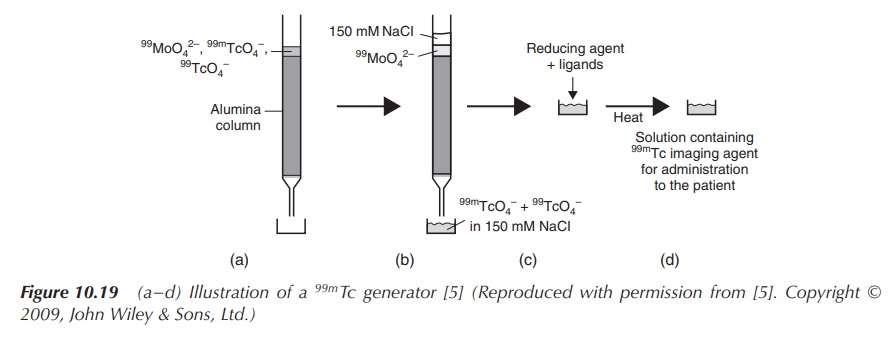
Figure 10.19 (a–d) Illustration of a 99mTc generator (Reproduced with permission from . Copyright © 2009, John Wiley & Sons, Ltd.)
Compounds containing 99mTc can be used for imaging a
variety of functions and structures in the human body. The use of different
molecules containing 99mTc determines to which part of the body the
radionu-clide is transported and which structure can be imaged. There are a
variety of different molecules, but, for example, 99mTc-aerosol can
be used for the imaging of lung ventilation, whereas 99mTc-albumin
is generally used for judging cardiac function. 99mTc-albumin is an
injectable solution prepared by combining sodium pertechnetate (NaTcO4)
and human albumin in the presence of a reducing agent such as a tin salt .
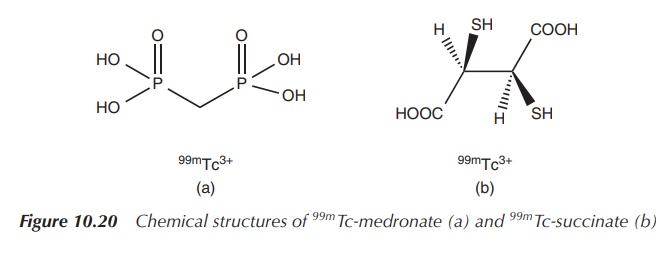
99mTc-medronate is used for skeletal imaging, and the succimer analogue is used for preparing images of the kidney. 99mTe succimer injection is prepared by reacting sodium pertechnetate (NaTcO4) with meso-2,3-dimercaptosuccinic acid in the presence of a reducing agent such as a stannous salt (Figure 10.20) .
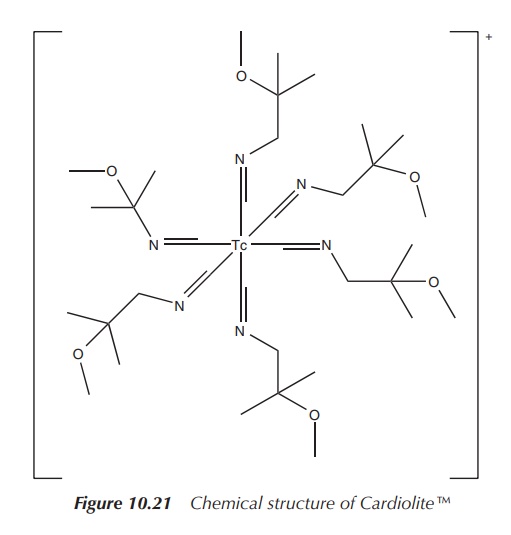
Cardiolite is an organometallic compound based on 99mTc,
which has become one of the most used nuclear imaging agents to visualise the
heart muscle and abnormalities of the parathyroid. Cardiolite is the trade name
of 99mTc-sestamibi, which is a coordination complex of 99mTc
with six so-called MIBI ligands. MIBI stands for methoxyisobutylisonitrile. The
full chemical name is (OC-6-11)-hexakis[1-(isocyano-
C)-2-methoxy-2-methylpropane][99mTc]technetium(I) chloride. A
typical solution for injection is prepared by heating a solution
tetrakis[(2-methoxy-2-methylpropyl-1-isocyanide)copper(I)] tetrafluoroborate,
which is a weak chelating agent, and sodium pertechnetate (NaTcO4)
in the presence of a stannous salt (Figure 10.21) .
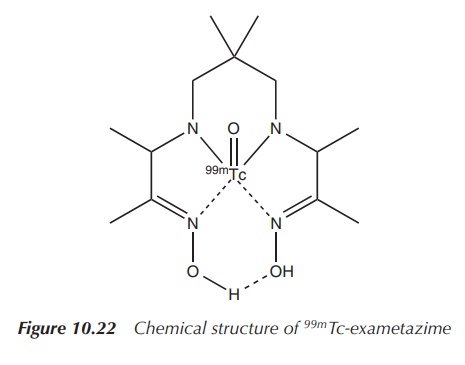
99mTc-exametazime is a 99mTc
preparation that can be used to visualise damage to the brain, for example, in
the evaluation and localisation of stroke damage, head trauma, dementia and
cerebral function impairment (Figure 10.22 and Table 10.5) .
Each of these 99mTc-containing compounds is freshly prepared by the radiopharmacist strictly following a standard protocol issued by the supplier. Usually, all ingredients are supplied in closed vials, mostly characterised as reagent vials, buffer vials and, if applicable, a vial containing stabiliser.
For illustration purposes, only the preparation
of 99mTc-exametazime for injection (as supplied by GE Healthcare) is
explained in the following. The nonstabilised formulation is prepared by adding
54 mCi of 99mTcO4− to a 5 ml reagent vial. The
reagent vial contains the racemic mixtures of the ligand exametazime [(3RS,9RS)-4,8-diaza-3,6,6,9-tetramethylundecane-2,10-dione
bisoxime] and stannous chloride dehydrate as reducing agent together with
sodium chloride . The preparation should have a pH of 9.0–9.8 and should be
used within 30 min .
2. 18Fluoride: PET scan
Fluorine has the chemical symbol F and atomic number 9 and is
the most electronegative element. It belongs to group 17 of the periodic table,
the so-called halogens. Fluorine typically exists as a diatomic molecule at
room temperature.
There are 18 isotopes known of fluorine, but only 1 (19F)
is stable. Most of the radioactive isotopes have a very short half-life, mostly
<1 min.
Only the radioisotope 18F has a longer half-life of around 110 min
and is clinically used (Figure 10.23).
18F is a
positron-emitting radioisotope and is used in radiopharmaceutical imaging such
as PET scanning. Two compounds, namely fluorodeoxyglucose (18F-FDG)
and derivatives of 18F choline, are under intense clinical
investigation and/or use.
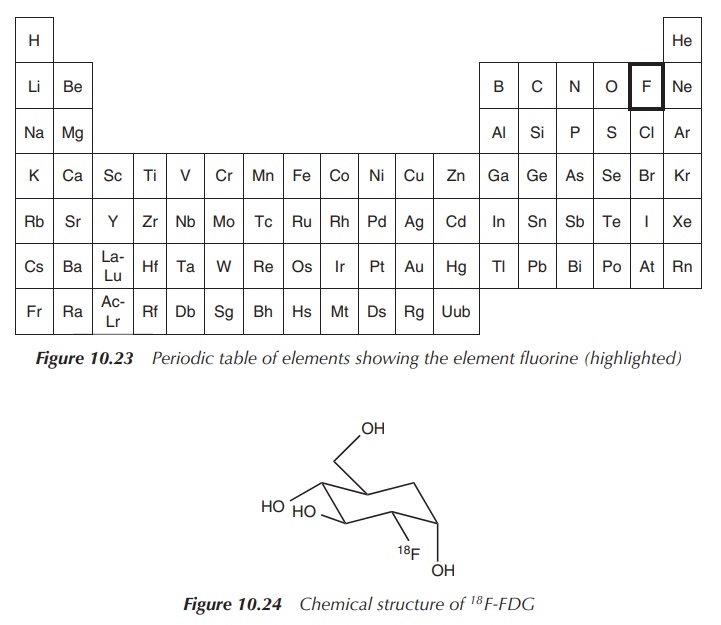
18F-FDG is a glucose
derivative that contains a radiolabel (18F) at the 2′
position replacing the hydroxyl group. 18F-FDG is administered
intravenously and is used as an assessment of problems with glucose metabolism,
especially in the brain, often associated with epilepsy and in cancer. Areas
where an increased absorption of 18F-FDG are visible correlate to
areas where an increased glucose metabolism is present. 18F-FDG is
distributed around the body similar to glucose and is cleared renally. There
are no known contraindications known to 18F-FDG (Figure 10.24).
18F-FDG is the main radioimaging agent used in PET scanning. Examples include studies of heart, where it is used to differentiate between dead and live tissue in order to assess the myocardium. In neurology, it can be used to diagnose dementia, seizure disorders or tumours of the brain. 18F-FDG is generally used to assess the extent of the tumour in a cancer patient. Cancerous tissue is characterised by increased cell proliferation, which requires energy, and therefore an increased amount of glucose. This leads to an accumulation of 18F-FDG in malignant tumours and allows judging the degree of metastasis formed. This information is important for any surgical procedure and also for the initial assessment of the cancer stage.
Unfortunately, there are limitations to the use of 18F-FDG,
as its uptake is not very specific. As a result, other conditions can also
cause an accumulation of 18F-FDG and can lead to misdiagnosis. These
conditions include inflammation and healing of wounds, which also show increased
glucose metabolism.
Therefore, a variety of other 18F-labelled compounds
are under intense scrutiny as alternative PET scanning agents, mainly compounds
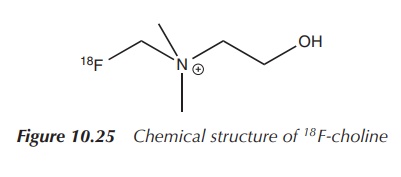
with a more specific biological pathway. This includes 18F-choline.
Choline is a compound incorporated into the cell membrane and therefore cells
dividing at a fast rate have an increased need for this substance. Studies for
a range of tumours were undertaken, but most studies focussed on prostate
cancer. In comparison to 18F-FDG, 18F-choline showed less
activity in the bladder and a prolonged elimination via the kidneys.
Additionally, biological processes other than cancer also include rapid
division of cells and can lead to misdiagnosis (Figure 10.25).
3. 67Gallium: PET
As previously mentioned (see Chapter 4),
gallium consist of two stable isotopes (69Ga and 71Ga)
and there are two radioisotopes (67Ga and 68Ga) that are
commercially available. 67Ga has a half-life of 3.3 days, whereas 68Ga
has an even shorter half-life of 68 min (Figure 10.26).
67Ga
decays via electron capture and subsequently emits γ-rays, which can be
detected with a gamma camera. 68Ga is a positron-emitting isotope
and is used for PET. Because of its short half-life, fresh 68Ga for
clinical applications is obtained through generators. The generator is equipped
with the parent compound 68Ge, which has a half-life of 271 days and
decays via electron capture to form the ‘daughter’ 68Ga.
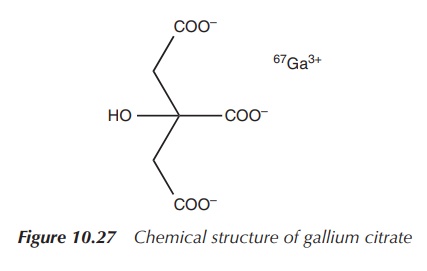
It has been reported that radioactive gallium-67 citrate
accumulates in malignant cells when injected into animals that are infected
with tumours. This has led to the development of 67Ga scans, which
have been used over the past two decades mostly for the detection of residual
cancer cells in patients with Hodgkin’s and non-Hodgkin’s lymphomas after chemo
or radiotherapy. The level of 67Ga present in lymphoma cells
correlates with their metabolic activity and directly with their proliferation
rate. Therefore, a positive 67Ga scan (mostly undertaken after
chemotherapy) indicates the survival of malignant cells and the need for
further treatment (Figure 10.27).
As previously mentioned (see Chapter 4), Ga3+ is
mainly transported by transferrin. In
vitro studies have shown that the uptake of the radioactive gallium into
the cancer cells was significantly increased when trans-ferrin was added to the
medium .
4. 201Thallium
The element thallium belongs to the boron group, and has the
chemical symbol Tl and atomic number 81.
Thallium is a soft grey metal, which cannot be found as the free
metal in nature (Figure 10.28).
The common oxidation states for thallium are +3, which resembles the oxidation states of other group members, and +1, which is actually the far more dominant oxidation state for thallium ions. Thallium ions with the oxidation state +1 follow alkali metals in their chemical behaviour and are handled in biological systems similar to potassium (K+) ions.
Thallium and many of its compounds are toxic. In particular, the
Tl+ cation displays good aqueous solubility and it can enter the
body via the potassium-based uptake processes as its behaviour is similar to
that of K+. Unfortunately, there are differences in the chemistry of
both ions that affect, for example, their binding to sulfur-containing
molecules and lead to the toxicity of thallium ions. Thallium-based compounds
were used as rat poison, but their use is nowadays discontinued as their toxic
properties are not very specific. Signs of thallium poisoning include hair
loss, nerve damage and, ultimately, at high enough doses, sudden death.
The radioactive thallium isotope 201Tl
was the main substance used for nuclear imaging in cardiology. It was used for
the so-called thallium nuclear cardiac stress test, where a radiotracer such as
201TlCl (thallous chloride-201) is injected into a patient during
exercise. After a short waiting period (in order to ensure good distribution of
the radioactive substance), images of the heart are taken with a gamma camera
and the blood flow within the heart muscle is evaluated. Nowadays, the radio
isotope has been mostly replaced by 99mTc imaging. The radio isotope
201Tl has a half-life of 73 h and can be generated using a
transportable thallium-201 generator. This generator uses 201Pb
(lead-201) as the ‘parent’, which decays via electron capture to the ‘daughter’
201Tl. 201Tl decays by electron capture and has good
imaging characteristics .
Related Topics
