Solved Problems on Acidity and Basicity
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Acidity and Basicity
Questions and answers, Solved Problems on Acidity and Basicity - Organic Chemistry : Acidity and Basicity
PROBLEMS
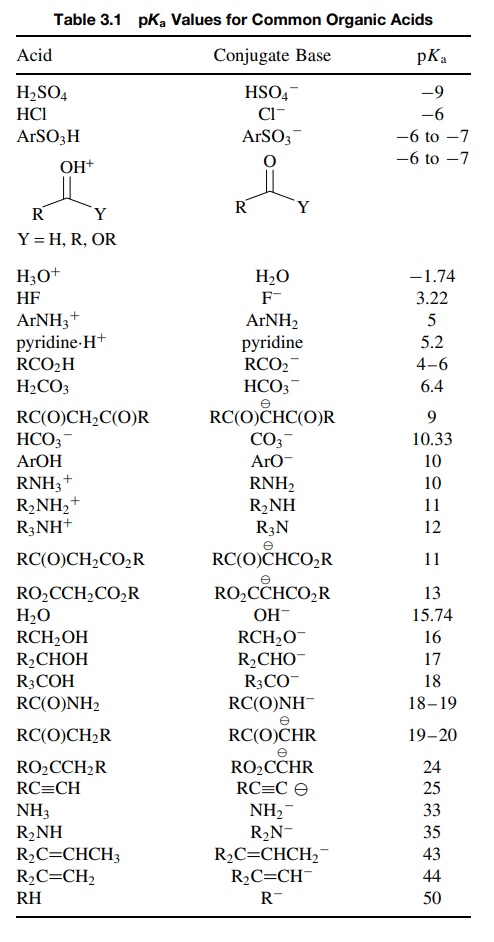
3.1. Using the pKa data from Table 3.1,
predict the position of the following equilibria and give an estimate of the
equilibrium constant:
(a) NH3 +
OH− ↔ NH2−
+ H2O
(b) CH3CO2H
+ CO3−2 ↔ CH3CO2−
+ HCO3−
(c) RCO2H
+ R2NH ↔ RCO2− + R2NH2+
(d) 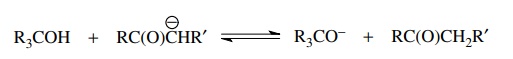
(e) NaF + HCl ↔ NaCl + HF
(f) ArOH + RNH2
↔
ArO− + RNH3+
(g) RC(O)OR′ + H2SO4
↔ RC(OH+)OR′ + HSO4−
(h) RC(O)CH2C(O)R′
+ R3N ↔ 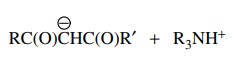
(i) RC ≡ C−
+ RCH2OH ↔ RC ≡ CH + RCH2O−
(j) CH3Li + (CH3)2C = CH2 ↔ CH4 + (CH3)2C=CHLi
Answer:
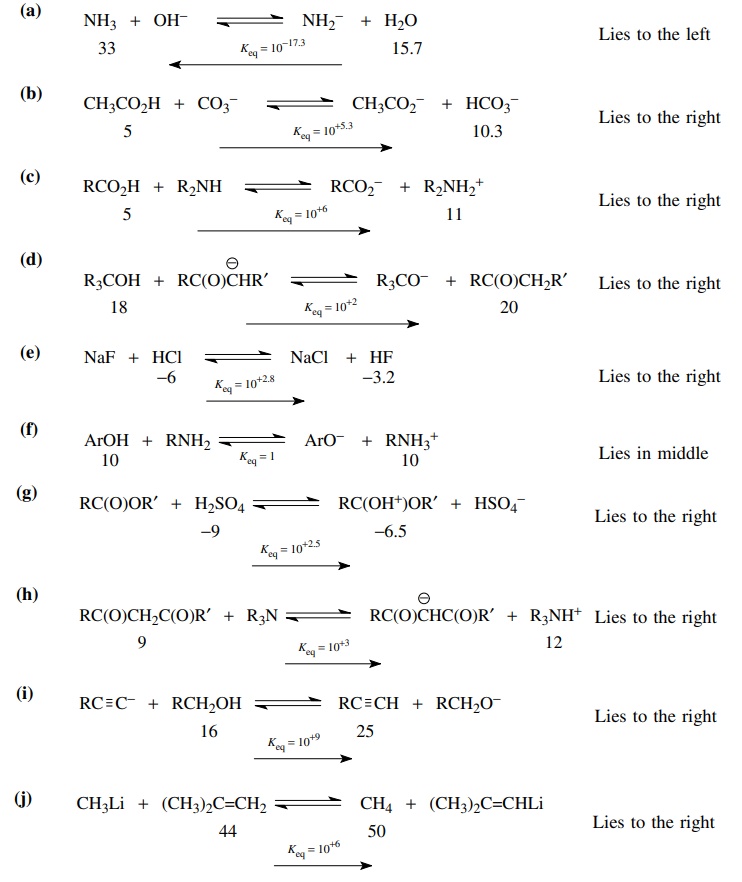
3.2. Predict the
positions of the following equilibria (left, right, or center) and give an
approximate pK value for the equilibrium. You may need to look up or estimate pKa values using structural
effects on the pKa values
of known compounds. If you do estimate the pKa’s,
rationalize your method of estimation.
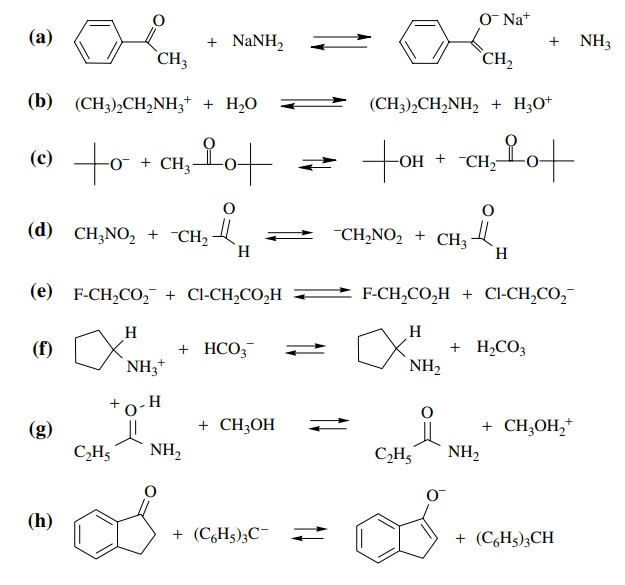
Answer:
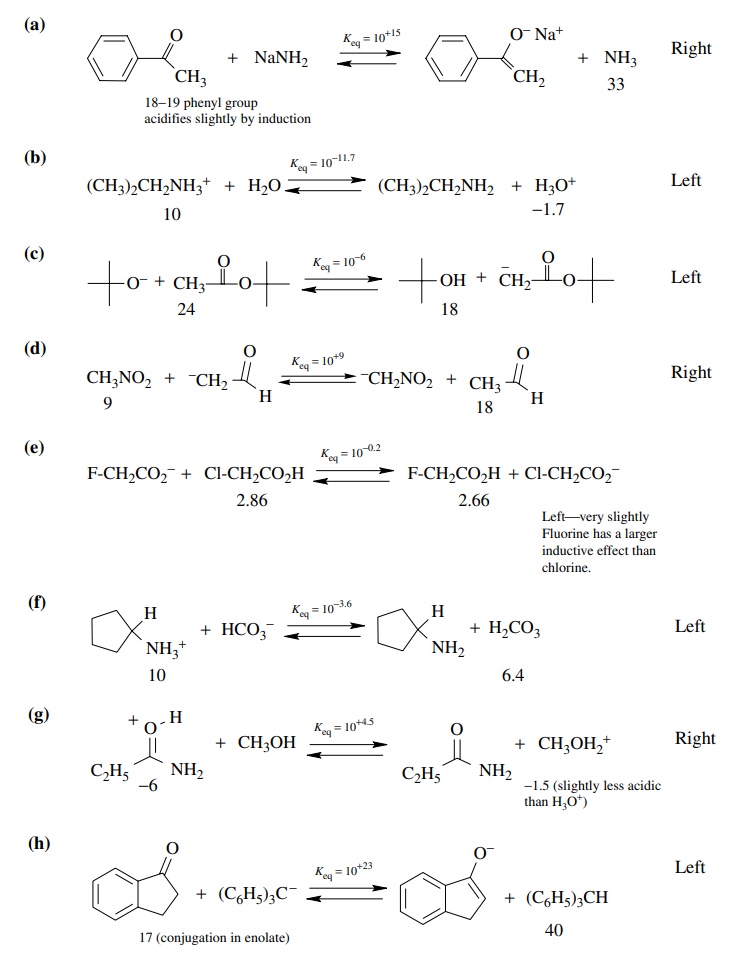
3.3. List (by
number) in order of increasing acidity of the underlined protons.
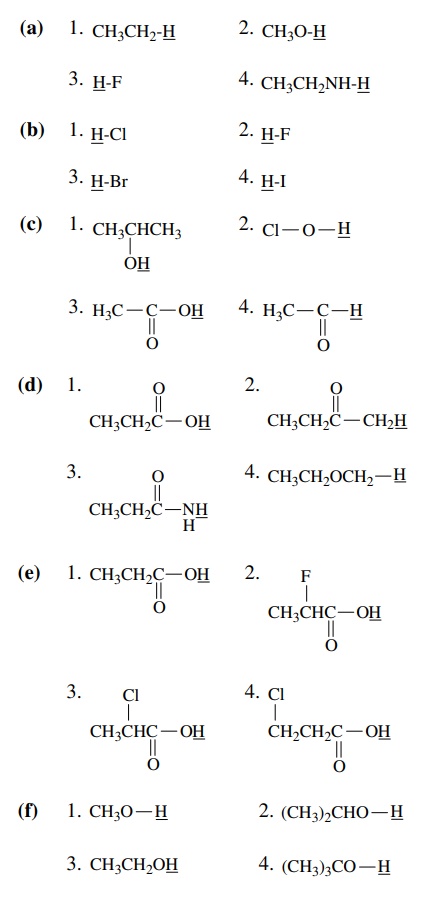

Answer:
(a)
1 < 4 < 2 < 3
(b)
2<1<3<4
(c)
4<1<2<3
(d)
4<2<3<1
(e)
1<4<3<2
(f)
4<2<3<1
(g)
1<4<3<2
(h)
3<2<1<4
(i)
1<3<2<4
(j)
4<1<2<3
(k)
4<2<3<1
3.4. Give
explanations for the following observations:
(a) Amides are
protonated on oxygen rather than on nitrogen.
(b) Ethers are
better Lewis bases than ketones.
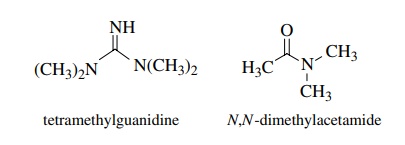
(c) Tetramethyl
guanidine (pKa ≈ 12) is a much
stronger base than N ,N - dimethylacetamide (pKa ≈ −0.5).
(d) Boron
trifluoride (BF3) is a stronger Lewis acid than trimethyl borate
[(CH3O)3B].
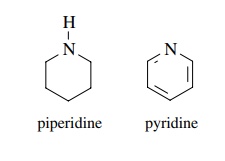
(e) Piperidine is a
much stronger Lewis base than pyridine.
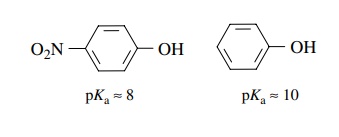
(f) p-Nitrophenol has pKa ≈ 8 whereas phenol has pKa ≈ 10.
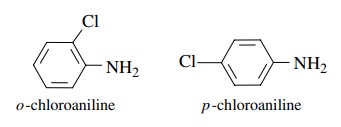
(g) o-Chloroaniline is a weaker base than p-chloroaniline.
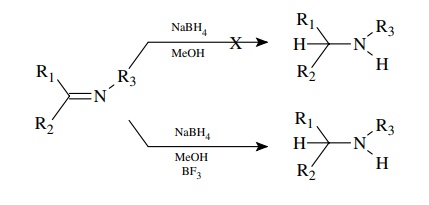
(h) Sodium
borohydride in alcohol does not reduce imines effectively. If BF3 is
added to the mixture, however, the reduction proceeds much more rapidly and
efficiently.
Answer:
(a)
Amides are protonated on oxygen because a resonance-stabilized cation is
produced. Protonation on nitrogen gives a localized cation.

(b)
The lone pairs on the ether oxygen are in sp3 while those of the
ketone are in sp2 orbitals. Thus the e− pairs of the ketone
are more tightly bound and less able to be donated, making the ketone the
weaker base.
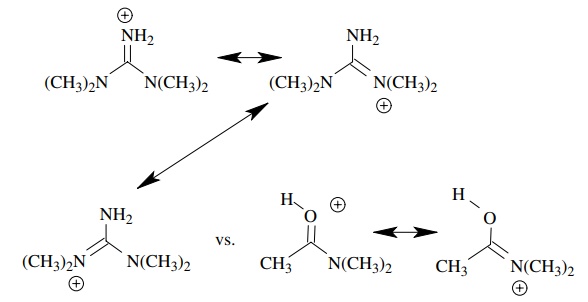
(c)
This is because the conjugate acid of tetramethylguanidine has much greater
resonance stabilization than the N ,N -dimethyl amide (more forms and
equiva-lent forms.)
(d)
The amount of e−
donation to boron by resonance is much greater for oxygen than it is for
fluorine because of fluorine’s greater electronegativity. Therefore the boron
of trimethyl borate is more e− rich and it is a poorer Lewis acid
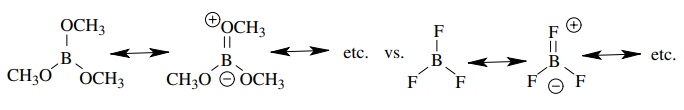
(e) The lone pair of piperidine is in an sp3
orbital while the lone pair of pyridine is in an sp2 orbital. The
greater s character causes the e− to be more tightly bound and less
able to be donated to H+
.
(f)
The conjugate base of p-nitrophenol
is resonance delocalized into the nitro group. The negative charge is thus more
stable and the conjugate acid is more acidic.
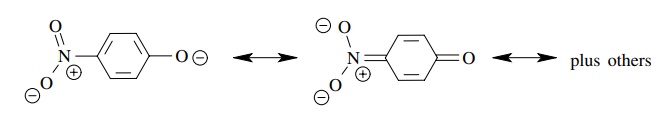
(g)
The inductive effect of the chlorine in the ortho position is greater than that
of the para chloro group because it is closer to the amino group. The greater
inductive effect removes more e− density from nitrogen and makes the
ortho isomer a weaker base.
(h) BF3 complexes with the lone pair of the imine and makes it much more electron deficient and thus electrophilic. In the complexed form it reacts with NaBH4 because it is a much better electrophile
3.5. Provide a rationale for the fact that hydrogen sulfide (H2S) is more acidic than water (H2O).
Answer:
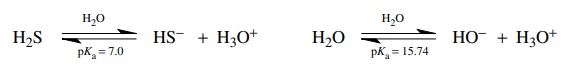
Oxygen
is more electronegative than sulfur so the hydroxide ion should be more stable
than the hydrosulfide anion. This should result in water being more acidic.
However, the hydrogen – sulfur bond is nearly 40 kcal/mol weaker than the
hydro-gen – oxygen bond. Thus the energy cost of bond breaking for hydrogen
sulfide is much less than for water. This overshadows the effect of
electronegativity and makes hydrogen sulfide more acidic.
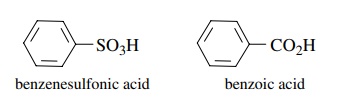
3.6. It has been
found that benzenesulfonic acid (pKa
≈ −6)
is a much stronger acid than benzoic acid (pKa ≈
5). Discuss the factors that can account for this.
Answer:
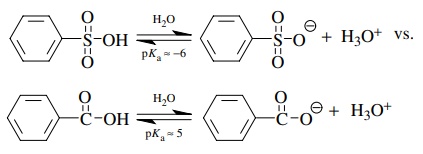
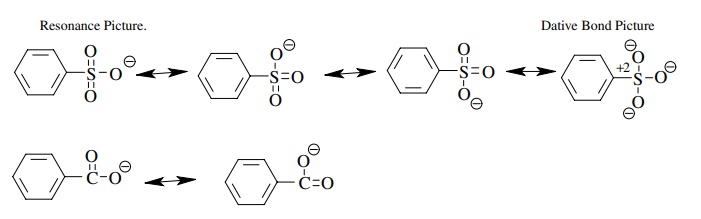
Compare
the conjugate bases. Since sulfur is slightly more electronegative than carbon
and is bonded to two other oxygens rather than one, the inductive effect
predicts benzenesulfonic acid should be stronger. But the huge difference in
acidity is much more than can be explained by an inductive effect argument. The
sulfonate ion has three equivalent resonance forms while the carboxylate group
has only two. This could explain the difference. However, the sulfur in such
systems is actually tetrahedral so any resonance argument must involve overlap
with the d orbitals on sulfur. Another bonding picture is two coordinate
covalent bonds from sulfur to oxygen. This leaves sulfur with a formal +2 charge right next to the anionic
oxygen. Since these are merely a different electron, this could simply be
considered an additional resonance form. Thus the greater resonance
stabilization of the sulfonate anion makes the conjugate acid very acidic.
3.7. The reaction of
pyrrole with a strong acid leads to protonation on C-2, not on nitrogen.
Explain why.
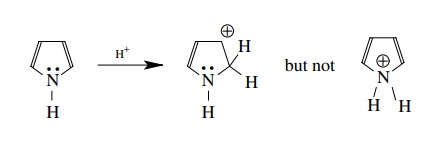
Answer:
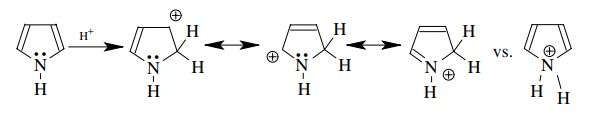
Protonation
at C-2 gives a resonance-stabilized (although nonaromatic) cation. Protonation
at nitrogen destroys the aromaticity of the pyrrole ring by taking the lone
pair out of the aromatic system. At the same time the cation produced is not
resonance stabilized. Thus protonation on C-2 is favored.
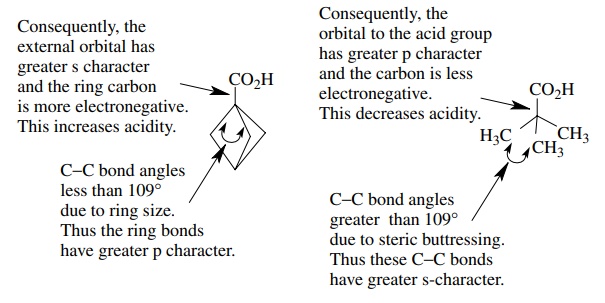
3.8. By pKa measurements it can be shown
that 1,1,1-bicyclopentanecarboxylic acid is 10 times more acidic than
2,2-dimethylpropanoic acid, even though both have a quaternary carbon attached
to the carboxylic acid group. Account for this difference in pKa.
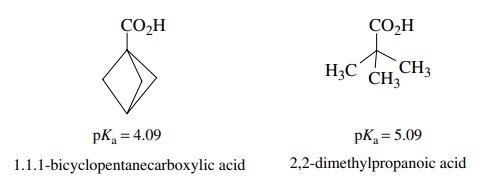
Answer:
3.9. It is observed
that the rate of ionization of allylic benzoates is, in most cases, inversely
proportional to the pKa’s
of the corresponding benzoic acids. (i.e., the lower the pKa, the faster the rate of ionization.) Account for this
correlation.
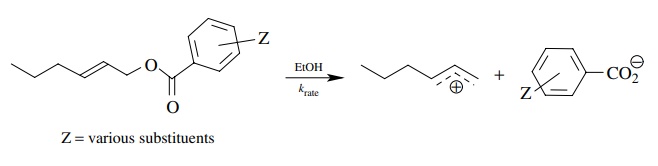
Answer:
Compare with
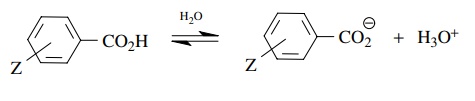
In
each case a benzoate ion is produced. Substituents which stabilize the benzoate
ion would make the benzoic acid more acidic and the pKa decreases. Substituents which stabilize the benzoate ion
should also cause it to ionize from the allyl group more easily (faster), and
thus the rate of ionization would go up. Thus substituents which make the
benzoate ion more stable cause the pKa
to go down and the rate of ionization to go up. The reverse is also true —
substituents which make the benzoate ion less stable would raise the pKa but lower the rate of
ionization. The two are thus inversely proportional.
Related Topics
