Antianginal Drugs
| Home | | Pharmacology |Chapter: Essential pharmacology : Antianginal and Other Anti-Ischaemic Drugs
Antianginal drugs are those that prevent, abort or terminate attacks of angina pectoris.
ANTIANGINAL DRUGS
Antianginal drugs are those that prevent, abort or terminate
attacks of angina pectoris.
Angina Pectoris
Is a pain syndrome due
to induction of an
adverse oxygen supply/ demand situation in a portion of the myocardium. Two
principal forms are recognized:
Classical angina (common form) Attacks
are predictably provoked
(stable angina) by exercise, emotion, eating or coitus and subside when the increased
energy demand is withdrawn. The underlying pathology is—severe arteriosclerotic
affliction of larger coronary arteries (conducting vessels) which run
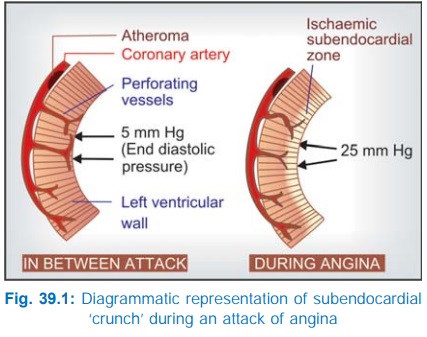
epicardially and send perforating branches to supply the deeper tissue (Fig.
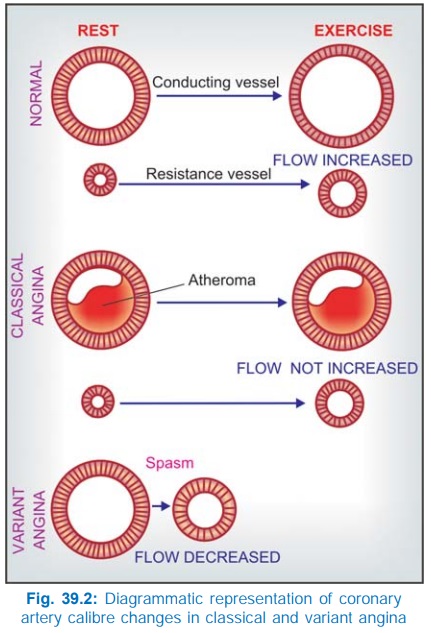
39.1). The coronary obstruction is ‘fixed’; blood flow fails to increase during
increased demand despite local factors mediated dilatation of resistance
vessels (Fig. 39.2) and ischaemic pain is felt. Due to inadequacy of ischaemic
left ventricle, the end diastolic left ventricular pressure rises from 5 to
about 25 mm Hg—produces sub-endocardial ‘crunch’ during diastole (blood flow to
the subendocardial region occurs only during diastole) and aggravates the
ischaemia in this region. Thus, a form of acutely developing and rapidly
reversible left ventricular failure results which is relieved by taking rest and
reducing the myocardial workload.
Drugs that are useful,
primarily reduce cardiac work (directly by acting on heart or indirectly by reducing
preload hence end diastolic pressure, and afterload). They may also cause
favourable redistribution of blood flow to the ischaemic areas.
Variant/Prinzmetal’s angina
(uncommon form)
Attacks occur at rest
or during sleep and are unpredictable. They are due to recurrent localized
(occasionally diffuse) coronary vasospasm (Fig. 39.2) which may be superimposed
on arteriosclerotic coronary artery disease.
Abnormally reactive
and hypertrophied segments in the coronary arteries have been demonstrated.
Drugs are aimed at preventing and relieving the coronary vasospasm.
Unstable angina with rapid increase in
duration and severity of attacks is
mostly due to rupture of an atheromatous plaque attracting platelet deposition
and progressive occlusion of the coronary artery; occasionally with associated
coronary vasospasm.
Chronically reduced
blood supply causes atrophy of cardiac muscle with fibrous replacement (reduced
myocardial work capacity → CHF) and may damage conducting tissue to
produce unstable cardiac rhythms. Antianginal drugs relieve cardiac ischaemia
but do not alter the course of coronary artery pathology: no permanent benefit
is afforded. On the other hand, aspirin, ACE inhibitors and statins (hypocholesterolaemic)
can modify coronary artery disease and improve prognosis.
Glyceryl trinitrate, the drug unsurpassed
in its ability to abort and terminate
anginal attack, was introduced by Murrell in 1879. Other organic nitrates were
added later, but a breakthrough was achieved in 1963 when propranolol was used
for chronic prophylaxis. The calcium channel blockers have been a major
contribution of the 1970s and continue to proliferate. A number of vasodilator
and other drugs have been promoted from time to time, but none is as uniformly
effective. Some potassium channel openers (nicorandil) and metabolic modulators
(trimetazidine, ranolazine) have been introduced lately.
Classification
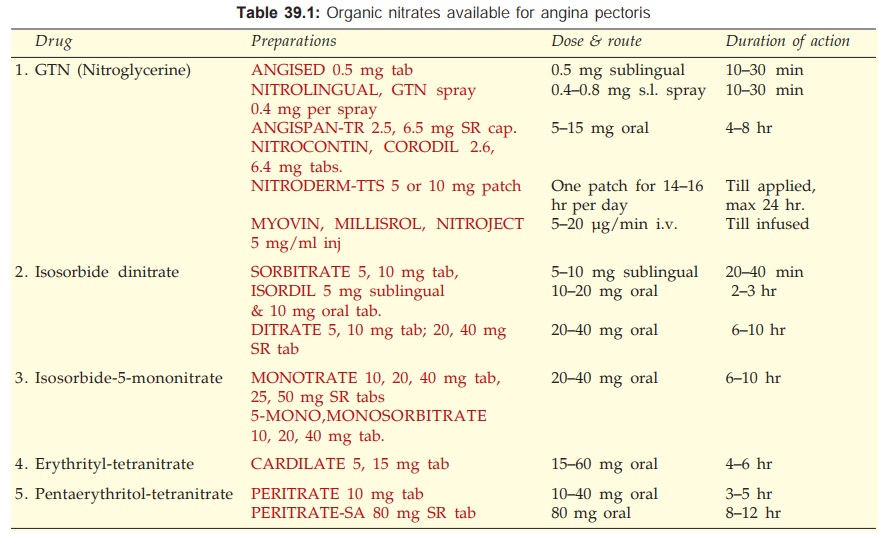
1. Nitrates
Short acting: Glyceryl trinitrate
(GTN, Nitroglycerine)
Long acting: Isosorbide dinitrate
(short acting by sublingual route),
Isosorbide mononitrate, Erythrityl tetranitrate, Pentaerythritol tetranitrate
2.
β Blockers Propranolol, Metoprolol, Atenolol and others.
3. Calcium Channel Blockers
Phenyl alkylamine:Verapamil
Benzothiazepine: Diltiazem
Dihydropyridines: Nifedipine,
Felodipine, Amlodipine, Nitrendipine, Nimodipine, Lacidipine, Lercanidipine,
Benidipine
4.
Potassium Channel Opener
Nicorandil
5. Others Dipyridamole,
Trimetazidine, Ranolazine, Oxyphedrine
Clinical Classification
A. Used To Abort Or Terminate Attack GTN,
Isosorbide dinitrate (sublingually).
B. Used For Chronic
Prophylaxis
All other drugs.
NITRATES ( (GTN as prototype)
All organic nitrates
share the same action; differ only in time course. The only major action is
direct nonspecific smooth muscle relaxation.
Preload Reduction The most prominent
action is exerted on vascular
smooth muscle. Nitrates dilate veins more than arteries → peripheral pooling of
blood → decreased venous
return i.e. preload on heart is reduced → end diastolic size and
pressure are reduced → decreased cardiac work according to Laplace relationship—which describes the
effectiveness of ventricular wall tension in elevating intraventricular
pressure and the extent to which fibre shortening results in systolic ejection.
Wall Tension =
intraventricular Pressure × ventricular Radius
Thus, reduction in ventricular
radius decreases the tension that must be generated in the ventricular
wall—hence decreased O2 consumption. Reduction in cardiac output
(c.o.) occurs at rest but is less marked during angina due to better
ventricular emptying. The decrease in end diastolic pressure abolishes the
subendocardial crunch by restoring the pressure gradient across ventricular
wall due to which subendocardial perfusion occurs during diastole. It is
through their action on peripheral veins that nitrates exert major beneficial
effects in classical angina.
Afterload Reduction Nitrates also produce
some arteriolar dilatation → slightly decrease
total peripheral resistance (t.p.r.) or afterload on heart; BP falls somewhat;
systolic more than diastolic (reflex sympathetic activity tends to maintain diastolic
BP). This action contributes to the reduction in cardiac work which is directly
proportional to aortic impedance.
With usual doses, and
if the patient does not stand still (which favours pooling of blood in the
legs), tachycardia is not prominent. With large doses and if the mean BP falls
significantly, reflex sympathetic stimulation occurs → tachycardia,
increased cardiac contractility → increased cardiac work → angina may be
precipitated. Fainting and cold sweat occur due to cerebral ischaemia. All
these can be prevented by lying down and raising the foot end.
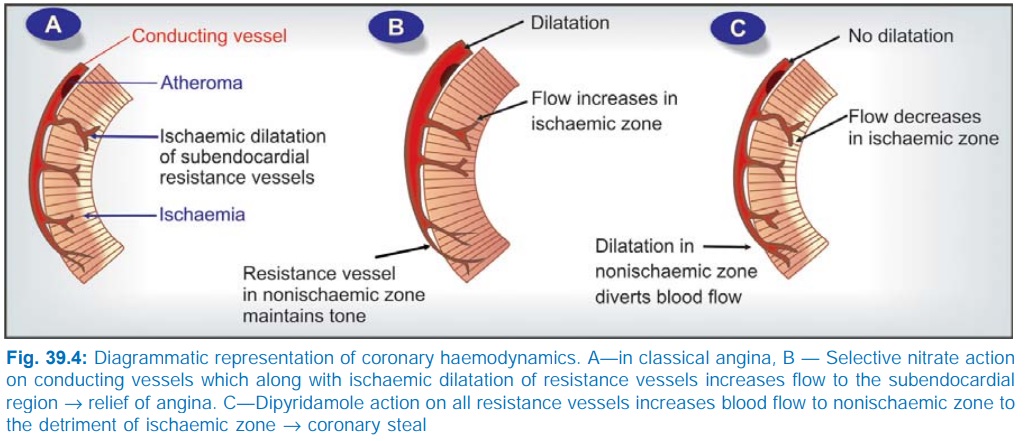
Redistribution Of Coronary Flow In the arterial tree, nitrates preferentially relax bigger
conducting (angiographically visible) coronary arteries than arterioles or
resistance vessels. This pattern of action may cause favourable redistribution
of blood flow to ischaemic areas in angina patients. Dilatation of conducting
vessels all over by nitrate along with ischaemia-induced dilatation of
autoregulatory resistance vessels only in the ischaemic zone increases blood
flow to this area, while in the non-ischaemic zones, resistance vessels maintain
their tone → flow does not
increase, or may decrease to compensate for increased flow to ischaemic zone.
In fact, nitrates do not appreciably increase total coronary flow in angina
patients.
Mechanism Of Relief Of Angina The dilator effect on larger coronary
vessels is the principal action of nitrates benefiting variant angina by
counteracting coronary spasm. In classical angina undoubtedly the primary
effect is to reduce cardiac work by action on peripheral vasculature, though
increased blood supply to ischaemic area may contribute. Exercise tolerance of
angina patients is increased because the same amount of exercise causes lesser
augmentation of cardiac work.
Heart And Peripheral Blood Flow Nitrates have no direct stimulant or depressant action on
the heart. They dilate cutaneous (especially over face and neck → flushing) and
meningeal vessels → headache. Splanchnic and renal blood flow decreases to
compensate for vasodilatation in other areas. Nitrates tend to decongest lungs
by shifting blood to systemic circulation.
Other Smooth Muscles Bronchi, biliary tract and esophagus are relaxed; effect on
intestine, ureter, uterus is variable and insignificant.
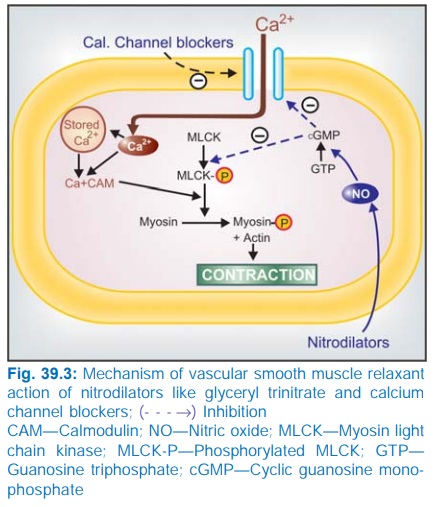
Mechanism of action
Organic nitrates are rapidly de-nitrated
enzymatically in the smooth muscle cell to release the reactive free radical nitric oxide (NO) which activates cytosolic guanylyl cyclase → increased cGMP → causes de-phosphorylation
of myosin light chain kinase (MLCK) through a cGMP dependent protein kinase
(Fig. 39.3). Reduced availability of phosphorylated (active) MLCK interferes
with activation of myosin it fails to interact with actin to cause contraction.
Consequently relaxation occurs. Raised intracellular cGMP may also reduce Ca2+
entry—contributing to relaxation.
Veins express greater amount of the enzyme that generates NO
from GTN than arteries—may account for the predominant venodilator action. It
has been indicated that preferential dilatation of epicardial conducting arteries
over autoregulatory arterioles is also due to differential distribution of
nitrate metabolizing enzymes in these vessels.
Platelets The NO generated from
nitrates activates cGMP production in
platelets as well, leading to a mild antiaggregatory effect. This action may be
valuable in unstable angina.
Pharmacokinetics
Organic nitrates are lipidsoluble: well absorbed from buccal
mucosa, intestines and skin. All except isosorbide mononitrate undergo
extensive and variable first pass metabolism in liver. They are rapidly
denitrated by a glutathione reductase and a mitochondrial aldehyde
dehydrogenase. The partly denitrated metabolites are less active, but have
longer t½. Though nitrates have been traditionally classified into short-acting
and long-acting, it is the rate of absorption from the site of administration
and the rate of metabolism that govern the duration of action of a particular
nitrate. For example, GTN and isosorbide dinitrate are both short-acting from
sublingual but longer-acting from oral route.
Adverse Effects
These are mostly due
to vasodilatation.
1.
Fullness in head, throbbing headache; some degree
of tolerance develops on continued use.
2.
Flushing, weakness, sweating, palpitation,
dizziness and fainting; these are mitigated by lying down and accentuated by
erect posture and alcohol.
3.
Methemoglobinemia: is not marked with
clinically used doses. However, it can reduce O2 carrying capacity
of blood in severe anaemia.
4.
Rashes are rare, though relatively more common
with pentaerythritol tetranitrate.
Tolerance Attenuation of haemodynamic and anti-ischaemic effect of nitrates occurs if
they are continuously present in the body. This tolerance weans off rapidly
(within hours) when the body is free of the drug. Clinically, no significant
tolerance develops on intermittent use of sublingual GTN for attacks of angina.
However, it may become important when GTN is used orally, transdermally or by
continuous i.v. infusion round the clock, as well as with the use of long
acting agents, especially sustained release formulations. Cross tolerance
occurs among all nitrates. Tolerance occurs more readily with higher doses.
The mechanism of nitrate tolerance is not well understood. Reduced
ability to generate NO due to depletion of cellular SH radicals has been
demonstrated experimentally. However, thiol replinishing agents only partially
overcome nitrate tolerance. This form of therapy has not met clinical success.
Other changes which interfere with NO production like inactivation of
mitochondrial aldehyde dehydrogenase could be involved. Activation of
compensatory mechanisms including volume expansion, sympathetic and renin-angiotensin
system stimulation or other humoral pathways as well as oxidative stress due to
free radicals generated during de-nitration may contribute to nitrate
tolerance.
The most practical way to prevent nitrate tolerance is to
provide nitrate free intervals everyday.
Dependence On organic nitrates is now well recognized. Sudden withdrawal after prolonged
exposure has resulted in spasm of coronary and peripheral blood vessels. MI and
sudden deaths have been recorded. Angina threshold may be lowered during
nitrate free interval in some patients: episodes of angina may increase. In
such cases a drug of another class should be added. Withdrawal of nitrates
should be gradual.
Interactions
Sildenafil causes
dangerous potentiation of
nitrate action: severe hypotension, MI and deaths are on record. Additive hypotension is
also possible when nitrate is given to a patient receiving other vasodilators.
INDIVIDUAL DRUGS
Glyceryl trinitrate (GTN,
Nitroglycerine)
It is a volatile liquid
which is adsorbed on the inert matrix of the tablet and rendered nonexplosive.
The tablets must be stored in a tightly closed glass (not plastic) container
lest the drug should evaporate away. The sublingual route is used when
terminating an attack or aborting an imminent one is the aim. The tablet may be
crushed under the teeth and spread over buccal mucosa. It acts within 1–2 min
(peak blood level in 3–6 min) because of direct absorption into systemic
circulation (bypassing liver where almost 90% is metabolized).
Plasma t½ is 2 min,
duration of action depends on the period it remains available for absorption
from buccal mucosa. The remaining part of the tablet may be spit or swallowed
when no longer needed. A sublingual spray formulation has been recently marketed—acts
more rapidly than sublingual tablet. Hepatic metabolizing capacity can be
overwhelmed by administering a large dose (5–15 mg) orally. Sustained release
oral capsules containing much larger amounts of GTN can be used for chronic
prophylaxis.
Nitroglycerine is
readily absorbed from the skin. In the early 1970s, cutaneous application as
ointment was found to produce haemodynamic effects for 4–6 hours. A transdermal
patch in which the drug is incorporated into a polymer bonded to adhesive
plaster (see p. 9) has been developed
which provides steady delivery for 24 hours. It starts working within 60 min
and has a bioavailability of 70–90%. However, development of tolerance and
dependence may jeopardise its value. It is advised that the patch be taken off
for 8 hours daily. A transmucosal dosage form which has to be stuck to the gums
under the upper lip has also been produced—acts in 5 min and releases the drug
for 4–6 hours.
Intravenous infusion
of GTN provides rapid, steady, titratable plasma concentration for as long as
desired. It has been successfully used for unstable angina, coronary vasospasm,
LVF accompanying MI, hypertension during cardiac surgery, etc. Begin with 5 μg/min, adjust
according to need. Early institution of infusion may limit the size of infarct
in MI.
Isosorbide dinitrate
It is a solid but
similar in properties to GTN;
can be used sublingually at the time of attack (slightly slower in action than
GTN, peak in 5–8 min) as well as orally for chronic prophylaxis. Presystemic
metabolism on oral administration is pronounced and variable. The t½ is 40 min,
but sustained release formulation may afford protection for 6–10 hours. Last
dose should not be taken later than 6 PM to allow nitrate level to fall during
sleep at night.
Isosorbide mononitrate
This is an active metabolite of isosorbide dinitrate. When
administered orally it undergoes little first pass metabolism: bioavailability
is high, interindividual differences are minimal and it is longer acting (t½
4–6 hr). Last dose is to be taken in the afternoon; SR tablet once a day in the
morning.
Erythrityl
tetranitrate and pentaerythritol tetranitrate
These are longer-acting
nitrates used only for chronic
prophylaxis. Sustained release oral preparations are now available for 2–3
times a day dosing. There has been considerable scepticism in the past about
the efficacy of orally administered long-acting nitrates. Studies with high
doses have shown that first-pass metabolism in liver can be saturated and
haemodynamic effects lasting 4–6 hours do occur.
Uses
Angina Pectoris
Nitrates are effective in classical as well as
variant angina. For aborting or terminating an attack, sublingual GTN tablet or
spray, or isosorbide dinitrate is taken on ‘as and when required’ basis. GTN
produces relief within 3 min in 75% patients, the rest may require another dose
or take longer (upto 9 min). Nitrates increase exercise tolerance and postpone
ECG changes of ischaemia. Longeracting formulations (oral, transdermal) of GTN
or other nitrates are used on regular schedule for chronic prophylaxis.
However, development of tolerance and dependence may limit the usefulness of
this approach: 6–8 drug free hours daily are advisable.
Acute Coronary Syndromes
These are characterized by rapid
worsening of anginal status of the patient : include unstable angina (UA) and
non-ST segment elevation myocardial infarction (NSTEMI). It needs aggressive
therapy with a combination of drugs intended to prevent further coronary
occlusion, increase coronary blood flow and decrease myocardial stress (oxygen
demand). Nitrates are useful by decreasing preload (myocardial work) as well as
by increasing coronary flow (dilatation and antagonism of coronary spasm, if
present). Initially GTN is given sublingually, but if pain persists after 3
tablets 5 min apart, i.v. infusion of GTN is started. The role of nitrates
appears to be limited to relief of pain, because no mortality benefit has been
demonstrated in large randomized clinical trials such as GISSI3 (1994) and ISIS4
(1995).
Antiplatelet drugs like aspirin, clopidogrel, GPIIb/IIIa
antagonists, with or without heparin are the primary measures in UA/NSTEMI. The
β blockers are
indicated in all patients (if there are no contraindications) to reduce
myocardial oxygen demand. A CCB is indicated only when coronary spasm is not
effectively counteracted by the nitrate. Revascularization by thrombolytics/coronary
angioplasty with stents/coronary bypass surgery is considered in high risk
patients.
Myocardial Infarction (MI)
Administered by carefully titrated i.v. infusion to avoid hypotension
and tachycardia, GTN is frequently used during evolving MI with the aim of relieving
chest pain, pulmonary congestion and limiting the area of necrosis by
favourably altering O2 balance in the marginal partially ischaemic
zone (a consequence of preload reduction). However, the evidence that it
decreases mortality is not robust; prognostic benefits appear marginal. Proper
patient selection is important. GTN should not be administered if:
·
Systolic BP is < 90 mm Hg
·
Heart rate is < 50 or > 100 beats/min
·
Right ventricular infarction is suspected
·
Hypotension caused by nitrate limits the administration
of β blockers which have
more powerful salutary effects.*
·
Patient has taken sildenafil in the past 24
hours.
CHF and Acute
LVF
The role of
vasodilators in CHF is described in
Ch. No. 37. Nitrates afford relief by venous pooling of blood (which can be aided
by sitting posture while managing acute LVF or severe chronic CHF) → reduced venous return
(preload) → decreased end
diastolic volume → improvement in left ventricular function by Laplace
law and regression of pulmonary
congestion. Intravenous GTN is the preparation of choice for emergency use:
rate of infusion must be guided by continuous haemodynamic monitoring.
Biliary Colic due to disease or morphine— responds to sublingual GTN or isosorbide dinitrate.
American Heart
Association/American College of Cardiology guidelines for the management of
patients with acute myocardial infarction. Circulation
2004, 110, 588636.
Esophageal
Spasm
Sublingual GTN promptly
relieves pain. Nitrates taken before a meal facilitate feeding in esophageal
achalasia by reducing esophageal tone.
Cyanide Poisoning
Nitrates generate
methaemoglobin which has high affinity for cyanide radical and forms cyano-methaemoglobin.
However, this may again dissociate to release cyanide. Therefore, sodium
thiosulfate is given to form Sod. thiocyanate which is poorly dissociable and
is excreted in urine.
Cytochrome and other
oxidative enzymes are thus protected from cyanide; even that which has
complexed CN is reactivated. However, early treatment is critical. The
antidotes should be repeated as required.

Sodium nitrite is used
for this purpose because it is a very weak vasodilator; large doses (>300
mg) sufficient to generate enough methaemoglobin can be injected i.v. without
producing hypotension.
β BLOCKERS
These drugs do not
dilate coronaries or other blood vessels; total coronary flow is rather reduced
due to blockade of dilator β2 receptors. However,
flow to the ischaemic subendocardial area is not reduced because of favourable
redistribution and decrease in ventricular wall tension. They act by reducing
cardiac work and O2 consumption (decreased heart rate, inotropic
state and mean BP). This is marginal at rest. More importantly, blockers limit
increase in these modalities that occurs during exercise or anxiety (due to
antiadrenergic action on heart).
All β blockers are nearly
equally effective in decreasing frequency and severity of attacks and in
increasing exercise tolerance in classical angina, but cardioselective agents
(atenolol, metoprolol) are preferred over nonselective β1 + β2 blockers (e.g. propranolol),
which may worsen variant angina due to unopposed α receptor mediated
coronary constriction that may accentuate the coronary spasm. Long term β blocker therapy
lowers risk of sudden cardiac death among ischaemic heart disease patients.
In angina pectoris, βblockers are to be
taken on a regular schedule; not on ‘as and when required’ basis. The dose has
to be individualized. Abrupt discontinuation after chronic use may precipitate
severe attacks, even MI.
Unstable Angina (UA)/Non-STelevation MI (NSTEMI): Unless contraindicated, β blockers are routinely used in UA/NSTEMI. However, they should be given only
after starting nitrate ± calcium channel blocker to counteract coronary vasospasm,
if present (β blockers carry the
risk of worsening coronary vasospasm). β blockers reduce
myocardial O2 demand and afford additional benefit by reducing risk
of impending MI/sudden cardiac death.
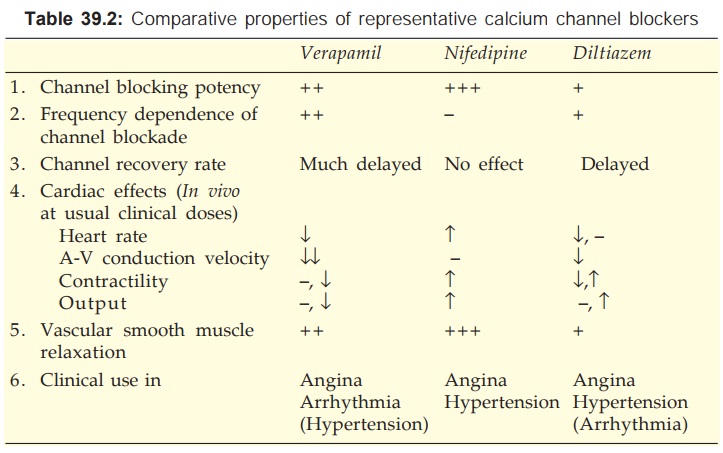
CALCIUM CHANNEL BLOCKERS
Verapamil was developed
in Germany in 1962 as a coronary dilator. It had additional cardio-depressant
property, but its mechanism of action was not known. Fleckenstein (1967) showed
that it interfered with Ca2+ movement into the cell. In the subsequent years, a
large number of chemically diverse Ca2+ channel blockers (CCBs) with different
pharmacological profiles have been produced.
Three important
classes of calcium channel blockers are examplified by:
Verapamil—a phenyl alkylamine, hydrophilic
papaverine congener.
Nifedipine—a dihydropyridine (lipophilic).
Diltiazem—a
hydrophilic benzothiazepine.
The dihydropyridines
(DHPs) are the most potent Ca2+ channel blockers, and this subclass has
proliferated exceptionally.
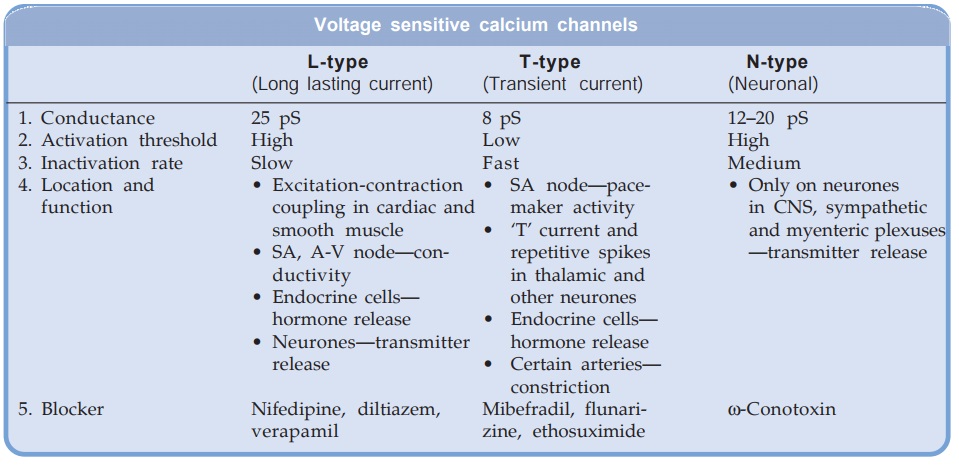
Calcium Channels
Three types of Ca2+ channels
have been described in smooth muscles (other excitable cells as well):
(a) Voltage Sensitive Channel Activated when membrane potential
drops to around –40 mV or lower.
(b) Receptor
Operated Channel Activated by Adr and other agonists—independent of membrane
depolarization (NA contracts even depolarized aortic smooth muscle by promoting
influx of Ca2+ through this channel and releasing Ca2+ from sarcoplasmic reticulum).
(c) Leak Channel
Small amounts of Ca2+ leak into the resting cell and are pumped out by Ca2+ATPase.
Mechanical stretch
promotes inward movement of Ca2+, which may be occurring through activation of
the leak channel or through separate stretch
sensitive channel.
The voltage sensitive Ca2+
channels are heterogeneous: three
major types have been identified.
All voltage sensitive
Ca2+ channels are membrane spanning funnel shaped glycoproteins that function
as ion selective valves. They are composed of a major α1 subunit which encloses
the ion channel and other modulatory subunits like α2,β, γ and δ. In L-type Ca2+
channels each subunit exists in multiple isoforms which may be site specific,
e.g.
Skeletal muscle L-channels
are:
α1s . α2/δa . β1 . γ
Cardiac muscle L-channels
are:
α1ca . α2/δc . β2
Smooth muscle L-channels
are:
α1cb . α2/δ . β3
Even smooth muscle L-channels
differ between vascular and nonvascular. Moreover distribution may be heterogeneous
in different parts of the vascular bed.
Only the voltage sensitive
L-type channels are blocked by the CCBs. The 3 groups of CCBs viz. phenylalkylamines (verapamil),
benzothiazepine (diltiazem) and dihydropyridines (nifedipine) bind to their own
specific binding sites on the α1 subunit; all restricting
Ca2+ entry, though characteristics of channel blockade differ. Further,
different drugs may have differing affinities for various site specific
isoforms of the L-channels. This may account for the differences in action
exhibited by various CCBs. The vascular smooth muscle has a more depolarized
membrane (RMP about –40 mV) than heart. This may contribute to vascular
selectivity of certain CCBs.
Pharmacological Actions And Adverse Effects
The common property of
all three subclasses of CCBs is to inhibit Ca2+ mediated slow channel component
of action potential (AP) in smooth/ cardiac muscle cell. The two most important
actions of CCBs are:
·
Smooth muscle (especially vascular)
relaxation.
·
Negative chronotropic, inotropic and dromotropic
action on heart.
Smooth Muscle
Smooth muscles
depolarise primarily by inward
Ca2+ movement through voltage sensitive channel. These Ca2+ ions trigger
release of more Ca2+ from intracellular stores and together bring about
excitation contraction coupling through phosphorylation of myosin light chain
as depicted in Fig. 39.3. CCBs cause relaxation by decreasing intracellular
availability of Ca2+. They markedly relax arterioles but have mild effect on
veins. Extravascular smooth muscle (bronchial, biliary, intestinal, vesical,
uterine) is also relaxed.
The dihydropyridines
(DHPs) have the most marked smooth muscle relaxant and vasodilator action;
verapamil is somewhat weaker followed by diltiazem.
Nitrendipine and other DHPs have been shown to release NO from endothelium and inhibit cAMP-phosphodiesterase resulting in raised smooth muscle cAMP. These additional mechanisms may account for their predominant smooth muscle relaxant action. Released endothelial NO may exert anti-atherosclerotic action.
Heart In the working atrial and ventricular fibres, Ca2+ moves in during plateau phase of
AP →releases more Ca2+ from sarcoplasmic
reticulum →contraction through binding
to troponin— allowing interaction of myosin with actin. The CCBs would thus
have negative inotropic action.
The 0 phase
depolarization in SA and AV nodes is largely Ca2+ mediated. Automaticity and
conductivity of these cells appear to be dependent on the rate of recovery of
the Ca2+ channel.
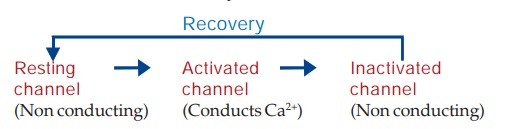
The L-type Ca2+
channels activate as well as inactivate at a slow rate. Consequently, Ca2+ depolarized
cells (SA and AV nodal) have a considerably less steep 0 phase and longer
refractory period. The recovery process which restores the channel to the state
from which it can again be activated by membrane depolarization is delayed by
verapamil and to a lesser extent by diltiazem (resulting in depression of
pacemaker activity and conduction), but not by DHPs (they have no negative
chronotropic/dromotropic action). Moreover, channel blockade by verapamil is
enhanced at higher rates of stimulation, that by nifedipine is independent of
frequency, while diltiazem is intermediate. Thus, verapamil slows sinus rate
and AV conduction, but nifedipine does not. Effect of diltiazem on sinus node
automaticity and AV conduction is similar to that of verapamil.
The relative potencies to block slow channels in smooth muscle do not parallel those in the heart. The DHPs are more selective for smooth muscle L-channels: at concentrations which cause vasodilatation they have negligible negative inotropic action. Diltiazem causes less depression of contractility than verapamil. Important differences between the three representative CCBs are summarized in Table 39.2. Their cardiac electrophysiological effects are compared in Table 38.1.
Verapamil
It dilates arterioles
and has some α adrenergic blocking
activity—decreases t.p.r. but BP is only modestly lowered. The pronounced
direct cardio-depressant effect is partially offset in vivo by reflex effects of peripheral vasodilatation. The HR
generally decreases, AV conduction is slowed, but c.o. is maintained by reflex
sympathetic stimulation and reduction in aortic impedance. However, ventricular
contractility may be markedly impaired in CHF patients. Coronary flow is
increased.
Dose: 40–160 mg TDS oral, 5 mg by slow i.v.
injection. CALAPTIN 40, 80 mg
tabs, 120, 240 mg SR tabs, 5 mg/ 2 ml inj.
Adverse Effects
Nausea, constipation
and bradycardia are more
common than other CCBs, while flushing, headache and ankle edema are less
common. Hypotension is occasional and tachycardia (common with DHPs) is absent.
It can accentuate conduction defects (contraindicated in 2nd and 3rd degree AV
block) and precipitate CHF in patients with preexisting disease. Cardiac arrest
has occurred on i.v. injection and when it is given to patients with sick
sinus.
Interactions
Verapamil should not
be given with β blockers—additive
sinus depression, conduction defects or asystole may occur.
It increases plasma
digoxin level by decreasing its excretion: toxicity can develop.
It should not be used
with other cardiac depressants like quinidine and disopyramide.
Diltiazem
It is a less potent
vasodilator than nifedipine and
verapamil, and has modest direct negative inotropic action, but direct
depression of SA node and AV conduction are equivalent to verapamil. Usual
clinical doses produce consistent fall in BP with little change or decrease in
HR. Large dose or i.v. injection decreases t.p.r. markedly which may elicit
reflex cardiac effects. It dilates coronaries.
Dose: 30–60 mg TDS–QID oral; DILZEM, 30, 60 mg
tabs, 90 mg SR tab; 25 mg/5
ml inj; ANGIZEM 30, 60, 90, 120, 180 mg tab, DILTIME 30, 60 mg tab; 90, 120 mg
SR tab.
Adverse Effects
Incidence of side
effects is low, but the profile is
similar to verapamil. Like verapamil, it also increases plasma digoxin.
Diltiazem should not be given to patients with preexisting
sinus, AV nodal or myocardial disease. Only low doses should be given to
patients on β blockers.
Nifedipine
It is the prototype
DHP with a rapid onset and short
duration of action. The overriding action of nifedipine is arteriolar
dilatation → t.p.r. decreases, BP
falls. The direct depressant effect on heart requires much higher dose, but a
weak negative inotropic action can be unmasked after β blockade. As
discussed above, it does not depress SA node or AV conduction. Reflex
sympathetic stimulation of heart predominates → tachycardia,
increased contractility and c.o. (no decrease in venous return along with
lowering of afterload aid increase in c.o.). Coronary flow is increased.
Nifedipine has mild natriuretic action, but significant diuresis
does not occur.
Dose: 5–20 mg BD–TDS oral.
CALCIGARD, DEPIN, NIFELAT 5, 10 mg tab, also 10 mg, 20 mg S.R.
(RETARD) tab; NICARDIA 5, 10 mg tab; 10, 20, 30 mg SR tab.
Adverse Effects
Frequent side effects
are palpitation, flushing, ankle edema, hypotension, headache, drowsiness and
nausea. These are related to peaks of drug level in blood: can be minimized by
low starting dose, fractionation of dose or use of retard formulation.
Nifedipine has paradoxically increased the frequency of angina in some
patients. Higher mortality among post MI patients has been confirmed. However,
it has been safely administered with β blockers and digoxin.
By its relaxant effect on bladder nifedipine can increase urine voiding
difficulty in elderly males. It has also been reported to hamper diabetes
control by decreasing insulin release.
OTHER DIHYDROPYRIDINES (DHPS)
All DHPs have pharmacodynamic
profile similar to nifedipine; there are minor differences in organ selectivity
and major differences in pharmaco kinetic characteristics. The slower and
longer acting ones induce less reflex sympathetic stimulation. Tachycardia,
propensity to increase cardiac work, flushing, headache, dizziness are subdued.
They are currently favoured, particularly since increased mortality among postMI
patients has been reported with the regular short-acting nifedipine
formulation.
Felodipine
It differs from
nifedipine in having greater vascular
selectivity, larger tissue distribution and longer t½. The extended release
preparation is suitable for once daily administration.
Dose: 5–10 mg OD, max. 10 mg
BD.
FELOGARD, PLENDIL,
RENDIL 2.5, 5, 10 mg ER tab.
Amlodipine
Pharmacokinetically it
is the most distinct DHP. It has
complete but slow oral absorption: peak after 6 to 9 hr—the early vasodilator
side effects (palpitation, flushing, headache, postural dizziness) are largely
avoided. Because of less extensive and less variable first pass metabolism, its
oral bioavailability is higher and more consistent. Volume of distribution and
t½ are exceptionally long: diurnal fluctuation in blood level is small and
action extends over the next morning.
Dose: 5–10 mg OD; AMLOPRES, AMCARD,
AMLOPIN, MYODURA 2.5, 5, 10 mg
tabs.
S(–)Amlodipine The single enantiomer
preparation is effective at half the dose and is claimed to cause less ankle
edema.
Dose: 2.5–5 mg OD;
SNUMLO, SAMCARD,
ASOMEX, ESAM 2.5, 5 mg tabs.
Nitrendipine
A DHP with oral
bioavailability of 10–30% and elimination
t½ of 4–12 hours. It has been shown to release NO from the endothelium and
inhibit cAMP phosphodiesterase; which may be the additional mechanisms of
vasodilator action. The endothelial NO is claimed to retard atherosclerosis.
Ventricular contractility and AV conduction are not depressed. Nitrendipine is
indicated in hypertension and angina pectoris.
Dose: 5–20 mg OD; NITREPIN, CARDIF 10,
20 mg tabs.
Lacidipine
A highly vasoselective
newer DHP suitable for once
daily administration. It is claimed to attain higher concentration in vascular smooth
muscle membrane; approved only for use as antihypertensive.
Dose: 4 mg OD, increase to 6
mg OD if required.
LACIVAS, SINOPIL 2, 4 mg tabs.
Nimodipine
It is a short-acting DHP which penetrates bloodbrain
barrier very efficiently due to high lipid solubility. It selectively relaxes cerebral
vasculature; approved for prevention and treatment of neurological deficit due
to cerebral vasospasm following subarachnoid haemorrhage or ruptured congenital
intracranial aneurysms. Side effects are headache, flushing, dizziness, palpitation
and nausea.
Dose: 30–60 mg 4–6 hourly
for 3 weeks following subarachnoid
haemorrhage; VASOTOP, NIMODIP, NIMOTIDE 30 mg tab; 10 mg/50 ml inj.
Lercanidipine
Another DHP similar to
nifedipine, but with longer duration of action. Peak plasma concentrations occur
at 1.5–3 hrs; t½ is 5–10 hours. It is indicated in hypertension at a dose of
10–20 mg OD.
LEREZ, LERKA 10, 20 mg
tabs.
Benidipine
A long-acting DHP that owes its long duration of
action to slow dissociation from the DHP receptor on the smooth muscle cell. It
is indicated in hypertension and angina pectoris. It is marketed only in India
and Japan.
Dose: 4–8 mg OD; CARITEC 4, 8 mg tab.
Pharmacokinetics
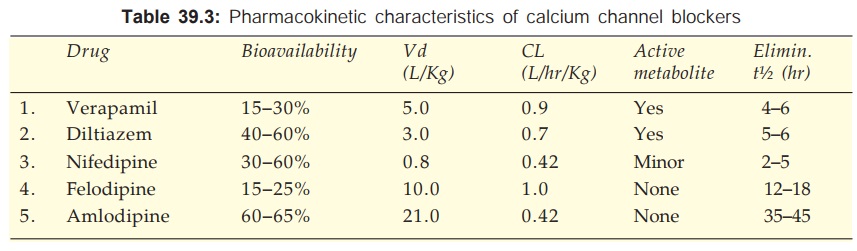
The pharmacokinetic
parameters of Ca2+ channel blockers are tabulated in Table 39.3. All are
90–100% absorbed orally, peak occurring at 1–3 hr (except amlodipine 6–9 hr).
The oral bioavailability of Ca2+ channel blockers is incomplete with marked
inter and intra individual variations. This is due to high first pass
metabolism (modest and less variable for amlodipine). All are highly plasma protein
bound (min.: diltiazem 80%, max.: felodipine 99%).
The Ca2+ channel
blockers are high clearance drugs with extensive tissue distribution. All are 90%
metabolized in liver and excreted in urine. Some metabolites are active. The
elimination t½ are in the range of 2–6 hr, but that of amlodipine is
exceptionally long; followed by lacidipine, nitrendipine and felodipine.
On chronic use
verapamil decreases its own metabolism—bioavailability is nearly doubled and t½
is prolonged.
Uses
Calcium channel blockers
can be safely given to patients with obstructive lung disease and peripheral
vascular disease in whom β blockers are contraindicated. The problem of
rebound worsening of angina on withdrawal after chronic use is less with CCBs
than with β blockers.
Angina Pectoris
All CCBs are effective
in reducing frequency and
severity of classical as well as variant angina. Benefit in classical
angina appears to be primarily due to
reduction in cardiac work: mainly as a result of reduced afterload. Though, they
can increase coronary flow in normal individuals, this is unlikely to be
significant in patients with fixed arterial obstruction. Exercise tolerance is
increased.
Many controlled studies and meta-analysis have concluded that
myocardial ischaemia may be aggravated by short-acting DHPs. This may be due to
decreased coronary flow secondary to fall in mean arterial pressure, reflex
tachycardia and coronary steal. The direct cardiac effect of verapamil and
diltiazem to reduce O2 requirement and less marked sympathetic
stimulation makes them less likely to aggravate ischaemia.
Trials using high dose
regular short-acting nifedipine formulation have reported increased mortality
among MI patients. The sudden rush of sympathetic activity evoked by each dose
of these preparations has been held responsible for the deleterious effect. The
slow and long-acting DHPs do not share this disadvantage. There is some
evidence that verapamil and diltiazem reduce reinfarction and mortality in MI
patients (equal to that achieved by β blockers) with uncompromised ventricular
function.
Myocardial infarction: The concensus opinion is against use of CCBs in MI, but verapamil/ diltiazem may be
employed for secondary prophylaxis when β blockers are contraindicated.
The capacity of CCBs to prevent arterial spasm is undoubtedly
responsible for the beneficial effect in variant
angina. Reduction of cardiac
O2 demand would also work in the same direction. No significant
difference in efficacy among different CCBs has been noted in angina pectoris.
CCBs are not a first
line treatment of unstable angina; may be used as add on therapy to nitrates when coronary vasospasm is prominent and is not counteracted by
nitrate alone. Use of nifedipine/DHPs in non β blocked patients is
to be avoided.
Hypertension
DHPs, diltiazem and verapamil are among the first line drugs for
hypertension (see Ch. No. 40).
Cardiac Arrhythmias
Verapamil and diltiazem are highly effective in PSVT and for
control of ventricular rate in supraventricular arrhythmias (see Ch. No. 38).
Hypertrophic Cardiomyopathy
The negative inotropic action of
verapamil can be salutary in this condition.
Other Uses
Nifedipine is an
alternative drug for premature labour;
Verapamil has been used to suppress migraine and nocturnal leg cramps. The DHPs
reduce severity of Raynaud’s episodes.
RATIONAL DRUG COMBINATIONS
Along with any of the
drugs used for chronic prophylaxis of angina, sublingual short-acting nitrate
is allowed on ‘as and when’ required basis to abort and terminate anginal
attacks when they occur.
Of the three major
classes of antianginal drugs described above, generally one agent is used
initially; choice depends on the stage of disease, associated cardaic/other medical
conditions and individual acceptability of side effects, because long-term
prognostic benefit and tolerability of long-acting nitrates (including
transdermal GTN), β blockers and long-acting CCBs is similar. However, some direct
comparative studies have found β blockers to achieve greater reduction in the
number of anginal attacks than CCBs, but objective measurements and outcome
were not different. When monotherapy is unable to provide adequate relief in
tolerated doses, concurrent use of 2 or 3 drugs may be tried.
I. β blocker + long-acting
nitrate combination is rational in classical angina because:
(a) Tachycardia due to
nitrate is blocked by β blocker.
(b) The tendency of β blocker to cause
ventricular dilatation is counteracted by nitrate.
(c) The tendency of β blocker to reduce
total coronary flow is opposed by nitrate.
II. The above advantages
may also be obtained by combining a slow acting DHP (in place of nitrate) with β blocker. However,
verapamil or diltiazem should not be used with β blocker since their
depressant effects on SA and AV node may add up.
III. Nitrates primarily
decrease preload, while CCBs have a greater effect on afterload. Their concurrent
use may decrease cardiac work to an extent not possible with either drug alone.
This combination may be especially valuable in severe vasospastic angina.
IV. In the most severe
and resistant cases of classical angina, combined use of all the three classes
is indicated. Since their primary mechanism of benefit is different, supra-additive
results may be obtained.
·
Nitrates primarily decrease preload.
·
CCBs mainly reduce afterload + increase
coronary flow.
·
β blockers decrease cardiac work primarily by
direct action on heart.
Verapamil/diltiazem
should be avoided in such combinations.
In randomized comparative studies, combinations have been found
superior to monotherapy only in more severe cases, but not in mild angina.
Recent evidence suggests a greater role of vasospasm of arteriosclerotic
segments of coronary arteries in precipitating attacks of angina. As such,
coronary dilator action of DHPs/nitrates may be more relevant.
POTASSIUM CHANNEL OPENERS
Minoxidil and
diazoxide are K+ channel openers which were used earlier in severe hypertension
and hypertensive emergencies. Novel K+ channel openers like nicorandil, pinacidil, cromakalim and
others have been developed in the 1990s.
Since intracellular
concentration of K+ is much higher (150 mM) compared to extracellular (4–5 mM),
K+ channel opening results in outflow of K+ ions and hyperpolarization. There
are multiple types of K+ channels, e.g. voltage dependent, Ca2+ activated,
receptor operated, ATP sensitive, Na+ activated and cell volume sensitive which
serve diverse functions and exhibit different sensitivities to drugs. As such,
K+ channel openers exhibit considerable diversity in action.
The most prominent
action of K+ channel openers is smooth muscle relaxation—vascular as well as
visceral: their potential clinical applications (see box) are primarily based on this property. Diazoxide and some
other K+ channel openers reduce insulin secretion, while sulfonylureas
(glibenclamide) cause hypoglycaemia by blocking K+ channels in pancreatic β cells and promoting
insulin release.
Potential Clinical
Applications Of K+ Channel Openers
·
Angina pectoris
·
Hypertension
·
Congestive heart failure
·
Myocardial salvage in MI
·
Antihypoglycaemic (Insulinoma)
·
Alopecia
·
Bronchial asthma
·
Urinary urge incontinence
·
Peripheral vascular disease (Raynaud’s,
cerebrovascular)
·
Erectile dysfunction
·
Premature labour
Nicorandil
This novel antianginal
drug activates ATP sensitive K+ channels— hyperpolarizing vascular smooth
muscle. The vasodilator action is partly antagonized by K+ channel blocker glibenclamide.
Like nitrates it also acts as a NO donor—relaxes blood vessels by increasing
cGMP. Thus, arterial dilatation is coupled with venodilatation. Coronary flow
is increased; dilatation of both epicardial conducting vessels and deeper
resistance vessels has been demonstrated. No significant cardiac effects on
contractility and conduction have been noted.
Beneficial effects on
angina frequency and exercise tolerance comparable to nitrates, β blockers and CCBs
have been obtained in stable as well as vasospastic angina. Mitochondrial K+ATP
channel opening by nicorandil is believed to exert myocardial protection by a
process of ischaemic preconditioning which
appears to reduce myocardial stunning, arrhythmias
and infarct size when a coronary
artery is suddenly blocked. Myocardial recovery from ischaemic damage after MI
as measured by left ventricular wall motion is improved by nicorandil.
The cardioprotective
property of nicorandil, has been supported by the large ‘Impact of nicorandil
in angina’ (IONA, 2002) randomized trial which found nicorandil to reduce acute
coronary events in high risk stable angina patients.
Side Effects are flushing,
palpitation, weakness, headache,
dizziness, nausea and vomiting. Large painful aphthous ulcers in the mouth,
which heal on stopping nicorandil have been reported.
Dose: 5–20 mg BD;
NIKORAN, 5, 10 mg tabs, 2 mg/vial,
48 mg/vial inj; KORANDIL 5, 10 mg tabs.
OTHER ANTIANGINAL DRUGS
Dipyridamole
It is a powerful
coronary dilator; increases total
coronary flow by preventing uptake and degradation of adenosine which is a
local mediator involved in autoregulation of coronary flow in response to
ischaemia. It dilates resistance vessels and abolishes autoregulation, but has
no effect on larger conducting coronary vessels. Cardiac work is not decreased
because venous return is not reduced. BP is minimally altered. It does not
afford symptomatic benefit or avert ECG changes of angina.
The pharmacological success but therapeutic failure of dipyridamole
has been explained on the basis of ‘coronary steal’ phenomenon (Fig. 39.4). By
dilating resistance vessels in non-ischaemic zone as well, it diverts the
already reduced blood flow away from the ischaemic zone.
Dipyridamole inhibits
platelet aggregation. By potentiating PGI2 and increasing cAMP in
platelets, it enhances antiaggregatory influences. Though not useful as an
antianginal drug, it is being employed for prophylaxis of coronary and cerebral
thrombosis in postMI and poststroke patients, as well as to prevent thrombosis
in patients with prosthetic heart valves (see
Ch. No. 44).
Dose: 25–100 mg TDS; PERSANTIN, CARDIWELL
25, 75, 100 mg tab.
Trimetazidine
This novel antianginal
drug acts by non-haemodynamic
mechanisms. There is no effect on determinants of myocardial O2 consumption,
such as HR and BP, both at rest as well as during exercise, but angina
frequency is reduced and exercise capacity is increased. In patients not
adequately controlled by long-acting nitrate/β blocker/CCB, addition
of trimetazidine further reduced anginal attacks and increased exercise
duration. The mechanism of action of trimetazidine is not known, but it may
improve cellular tolerance to ischaemia by:
Inhibiting mitochondrial long chain 3ketoacylCoAthiolase (LC3KAT)
a key enzyme in fatty acid oxidation—thereby reducing fatty acid metabolism and
increasing glucose metabolism in myocardium. Ischaemic myocardium shifts to
utilizing fatty acid as substrate, increasing requirement of O2 for
the same amount of ATP generated. Since oxidation of fatty acid requires more O2,
shift back of substrate to glucose would reduce O2 demand. It has
been labelled as pFOX (fatty acid oxidation pathway) inhibitor.
Limiting intracellular acidosis and Na+, Ca2+ accumulation
during ischaemia.
Protecting against O• free radical induced membrane damage.
Trimetazidine is absorbed orally, partly metabolized and largely
excreted unchanged in urine; t½ is 6 hr. It is generally well tolerated; side
effects are—gastric burning, dizziness, fatigue and muscle cramps. Reversible
parkinsonism has been reported in the elderly.
Trimetazidine has also been advocated for visual disturbances,
tinnitus, Méniére’s disease, dizziness, etc., but conclusive evidence of efficacy
in these conditions is lacking. For ischaemic heart disease, it has been widely
used in France, Spain, some other European countries and India, but not in the
UK or USA. It is mostly an add on medication to conventional therapy in angina
and postMI patients.
Dose: 20 mg TDS.
FLAVEDON 20 mg tabs, 35 mg modified release tab; CARVIDON,
TRIVEDON 20 mg tab.
Ranolazine
This recently
developed trimetazidine congener LC3KAT
inhibitor is a metabolic modifier approved by USFDA in 2006 for treatment of chronic
angina pectoris in patients who fail to respond to standard antianginal
therapy. Ranolazine spares fatty acid oxidation and shifts ATP production to
more O2 efficient carbohydrate oxidation. It also inhibits late INa
current in the myocardium which indirectly facilitates Ca2+ entry. Reduction in
Ca2+ overload in the myocardium during ischaemia may play an important role in
the cardioprotective action of ranolazine.
The efficacy of ranolazine in decreasing frequency of anginal
attacks and in prolonging exercise duration has been demonstrated both as
monotherapy as well as when added to conventional drugs (atenolol, amlodipine,
diltiazem) in multicentric randomized trials: MARISA (monotherapy assessment of
ranolazine in stable angina, 2004), CARISA (Combination assessment of
ranolazine in stable angina, 2004), ERICA (Efficacy of ranolazine in chronic
angina, 2005). Efficacy in acute coronary syndromes is being assessed in a
large (>6000 patients) study MERLINTIMI 36 (Metabolic efficiency with
ranolazine for less ischaemia in non ST elevation acute coronary syndromes).
Oral absorption of
ranolazine is slow taking 4–6 hours with a bioavailability of 30–50%. It is
metabolized in liver and excreted by the kidney with an average t½ of 7 hours.
Side effects reported are dizziness, weakness, constipation, postural
hypotension, headache and dyspepsia. Prolongation of QTc interval
has been noted; torsades de pointes is a risk, but not yet
encountered.
Dose: 0.5–1.0 g
BD; (marketed in USA as
ER tablet RANEXA)
Ranolazine is at present recommended in angina pectoris only in
combination with conventional therapy.
Oxyphedrine
This drug is claimed to improve myocardial metabolism
so that heart can sustain hypoxia better. Though used in angina and MI, its efficacy
and status in coronary artery disease is not defined. It can diminish or alter
taste sensation.
Dose: 8–24 mg TDS oral, 4–8
mg i.v. ODBD; ILDAMEN 8, 24 mg tab., 4 mg/2 ml inj.
Related Topics
