Assay of Antibiotics by Turbidimetric (or Nephelometric) Methods
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Microbiological (Microbial) Assays: Antibiotics-Vitamins- Amino Acids
A large number of antibioics, namely : chlortetracycline, doxycyline, gentamicin, neomycin, streptomycin, tobramycin and the like may be assayed tubidimetrically with fairly good accuracy.
ASSAY OF
ANTIBIOTICS BY TURBIDIMETRIC (OR NEPHELOMETRIC) METHOD
A large
number of antibioics, namely : chlortetracycline, doxycyline, gentamicin,
neomycin, streptomycin, tobramycin and the like may be assayed tubidimetrically
with fairly good accuracy.
1. Assay of Chlorotetracycline
Theory. Inoculate a medium consisting of
: peptone : 6 g, beef extract : 1.5 g, yeast extract : 3 g, sodium chloride : 3.5 g, D-glucose monohydrate : 1.0 g,
dipotassium hydrogen orthophosphate : 3.68 g, potassium hydrogen orthophosphate
: 1.32 g and dissolve in sufficient water to produce 1 L with a known quantity
of a suspension of Staphylococcus aureus
(NCTC 6571**) so as to obtain a readily measured opacity after an incubation of
about 4 hours. The micro-organisms must exhibit a sensitivity to the antibiotic
under investigation to such an extent that a sufficiently large inhibition of
growth takes place in the prevailing conditions of the test.
In actual
practice, it is always advisable that the inoculated medium should be used
immediately after its preparation. Using a phosphate buffer of pH 4.5 (dissolve
13.61 g of KH2 PO4 in about 750 ml of water, adjusting
the pH to 4.5 with 0.1 M NaOH and diluting to 1 L with water), prepare
solutions of the Standard Preparation and the substance under investigation at
concentrations presumed to be equal.
To enable
the validity of the assay to be examined, it is desirable to use at least three
doses of the Standard Preparation and of the substance being examined. It is
also advisable to use doses in logarith-mic progression in a parallel line
assay.
Materials Required : Standard
chlortertracyline ; sterilized media (as described above) : 1 L ; authentic and pure strain of
microorganism Staphylococcus aureus
(NCTC 6571) ; formaldehyde solu-tion (34–37% w/v) 10 mL ; matched identical
test tubes : 20 ;
Procedure : Distribute into identical
test-tubes an equal volume of standard tetracycline solution and the sample to be examined (having
presumed equal concentrations) and add to each tube an equal volume of
inoculated nutrient medium (for instance 1 mL of the solution and 9 ml of the
medium). Prepare at the same time two control tubes without the
chlorotetracycline, one containing the inoculated medium and the other
identical with it but treated immediately with 0.5 mL of formaldehyde solution.
These tubes are used to set the optical apparatus employed to measure the
growth.
Place all
the tubes, randomly distributed, in a water-bath or other suitable means of
bringing all the tubes rapidly to 35–37°C i.e.,
the incubation temperature and maintain them at that temperature for 3 to 4
hours, taking due precautions to ensure uniformity of temperatures and
identical incubation times. After incubation, stop the growth of the
microorganisms by adding 0.5 mL of formaldehyde solution, each tube and
subsequently measures the opacity to at least three significant figures using a
suitable optical apparatus. From the results calculate the potency of the
substance being examined i.e.,
chlortetracycline by standard statistical methods.*
Note. (a)
Rectilinearity** of the dose-response relationship, transformed or
untransformed, is often obtained only over a very limited range. It is this
range that must be used in calculating the activity and it must include at
least three consecutive doses in order to permit rectilinearity to be verified,
(b) Use in each assay the number of replications
per dose sufficient to ensure the required precision. The assay may be repeated
and the results combined statistically to obtain the required precision and to
ascertain whether the potency of the antibiotic being examined is not less than
the minimum required.
2. Cognate Assays
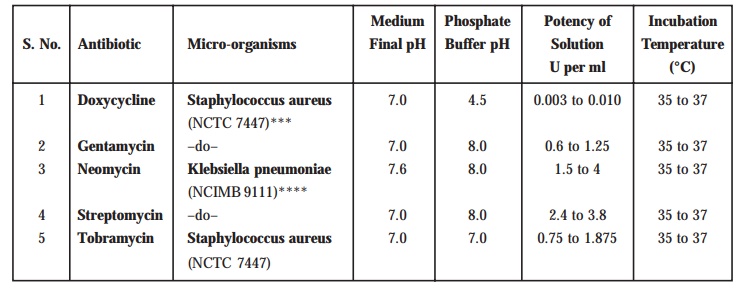
A few
other official antibiotics in BP (1993)
may also be assayed by adopting the method stated above, but using specific
micro-organism, definite final pH of the medium, pH of the phosphate buffer,
potency of solution (U per ml) an the incubation temperature. A few typical examples
are given in Table 10.6 below :
Table 10.6. Assay of Antibiotics Turbidimetrically
3. Assay of Vitamins
As or
late the judicious exploitation of various microorganisms as dependable and
reliable ‘analystical tools’ in a well
organized Quality Assurance Laboratory
(QAL) for the precise determi-nation of a plethora of Vitamins and amino acids.
Merit of Microbial Assays. There are
several well-known merits of microbial
assays as enu-merated under :
(1) These
are as precise and accurate as the ‘chemical
methods’.
(2) These
are invariably quite simple, convenient, not-so-cumbersome, and above all
definitely inexpensive.
(3) A
very small quantum of the ‘sample’
is required for the recommended microbial
assay.
(4) They
hardly need any elaborated instrumentation.
(5) These
microbial assays do require the
following essential criteria, such as :
·
ascertains continuous checks for consistency of
results,
·
ensures specificity, and
·
prevents any possible interferences.
(6) Automation of microbial assays may
essentially overcome any possible limitations, accu-racy of observations, and
the sample-handling capacity to a significant extent.
Example : In an ‘automatic photometric assay’ the following activities do take
place in a sequential manner, namely
:
·
measures the exact quantum of antibiotic present in
a given solution,
·
incorporates requisite quantum of inoculum and
nutrient medium,
·
incubates the resulting mixture for 100 minutes,
·
transfers the incubated mixture to photometer cell,
and
·
results are adequately read and recorded.
Principle. It has been amply proved and
established that there are some specific microbes which predominantly require vitamin
(factor) for their usual normal growth phenomenon ; and, therefore, are
quite sensitive to the extremely small quantities of the much desired ‘factor’. Nevertheless, it is
pre-cisely the critical inherent ability of these particular microorganisms (i.e., the ‘test organisms’) to carry out the synthesis of the ‘factor’ being determined. This
ultimately gives rise to the fundamental basis of the microbial assay of vitamins. To accomplish the
ultimate objective, the ‘test organism’
is duly inoculated in the highly specialized culture media that are essentially
complete in every possible re-spects except the presence of the ‘factor’ under investigative study. In
reality, it evidently caters for the ‘control’
wherein either little or almost minimal growth of microbes is duly
exhibited. Importantly, in another
set of parallel/identical experiments, one may incorporate meticulously the ‘graded quantities of factor’ the thus the ultimate growth of the test organism (i.e.,
response) is observed adequately. However,
one may observe invariably that the ‘response’
(i.e., growth of the ‘test organism’) is directly proportional
to the ‘factor’ (i.e.,quantum of the dose) actually
incorporated to the culture medium.
Microbial assays of the following three water-soluble vitamins would be
discussed individually in the
sections that follows :
(1) Calcium
Pantothenate,
(2) Niacin
(or Niacinamide), and
(3) Vitamin
B12 (or Cyanocobalamin).
(1) Calcium Pantothenate
It refers
to one of the B complex vitamins (or
vitamin B complex). The various
steps involved for the assay are enumerated under sequentially :
(1) Reagents. The various reagents essentially
required for the assay of ‘calcium
pantothenate’ are :
(a) Standardized Stock Solution. Each mL
of this stock solution consists of 50 mcg of cal-cium panthothenate. It
may be prepared by carefully dissolving 50 mg of BPCRS* calcium pantothenate
in 500 mL of double-distilled water ; 10 mL of 0.2 M acetic acid, 100 mL of
1.6% (w/v) sodium acetate ; and volume made upto 1 L with DW.
Note : The resulting solution must be stored under
a layer of ‘toluene’ in a refrigerator.
(b) Standard Solution. The standard solution should contain
approximately 0.04 mcg of cal-cium
pantothenate in 1 mL, and is duly
prepared by diluting the Standard Stock
Solution (a).
(c) Test Solution. The test solution essentially contains
nearly the same equivalent amount of calcium
pantothenate as present in the
Standard Solution (a) above i.e., 0.4 mcg.mL–1 prepared in double-distilled water.
(d) Culture Medium. The culture medium is composed of the following
solutions and ingredients :
Casein
hydrolysate solution** : 25 mL
Cysteine-tryptophane
solution : 25 mL
Polysorbate-80
solution*** : 0.25 mL
Dextrose
(anhydrous) : 10 g
Sodium
acetate (anhydrous) : 5 g
Adenine-guanine-uracil
solution : 5 mL
Riboflavin-Thiamine
hydrochloride-Biotin Solution : 5 mL
PABA****-Niacin-Pyridoxine
hydrochloride solution : 5 mL
Calcium
pantothenate solution A : 5 mL
Calcium
pantothenate solution B : 5 mL
The culture medium is usually prepared by
dissolving both anhydrous dextrose and sodium acetate in previously mixed
solutions and the pH is carefully adjusted to 6.8 with 1 M.NaOH solution. The
final volume is duly made upto 250 mL with distilled water and mixed
thoroughly.
(2) Stock Culture of Organism : The stock culture of organism may be
prepared dissolving 2 g
water-soluble yeast extract in 100 mL DW, 500 mg anhydrous dextrose, 500 mg
anhydrous sodium acetate, and 1.5 g agar. The resulting mixture is heated
gently so as to dissolve the agar. Now, 10 mL of hot solution is transferred to
test tubes and sterilized at 121°C by keeping in an upright position. The ‘stab culture’***** is now prepared
duly in three tubes employing Lactobacillus
plantarum, incubated at 30 to
37°C for 16 to 24 hours, and stored in a refrigerator ultimately.
(3) Preparation of Inoculum. The cells
consequently obtained from the stock
culture, (a) above, organism are duly transferred to a
sterile tube containing 10 mL of the culture emdium (d). Finally, it is incubated at 30 to 37°C for a duration of 16–24
hours.
(4) Methodology. The various steps involved are as
stated below :
(i) Standard Solution (b) is added to five test tubes in varying amounts viz., 1, 2, 3, 4 and 5 mL in
duplicate.
(ii) To
each of the five above test tubes
plus another four similar tubes
without any standard solution is added 5 mL of culture
medium, and the final volume made upto 10 mL with DW.
(iii) Now,
volumes of test solution (c) corresponding to either three or more of the levels as taken
above, are incorporated carefully to similar test tubes, in duplicate.
(iv) To
each test tube 5 mL of the medium solution, and volume is made upto 10 mL with DW.
Thus, we may have two separate racks
:
First Rack : Having complete set of standard plus
assay tubes ; and
Second Rack : Having duplicate set only.
(v) Tubes
of both the series are duly heated in an autoclave at 121°C for 5 minutes only ; cooled to ambient temperature, added 1
drop of inoculum (3) to each tube except two
of the four tubes that specifically
has no ‘standard solution’ (i.e., the uninoculated tubes), and mixed thoroughly. The tubes are adequately
incubated at 121°C at 30–37°C for 16– 24 hours.
(vi) Transmittance of the
various tubes is measured with a spectrophotometer
at wave-length ranging between 540–660
nm.
(5) Calculation. First of all, a standard concentration response curve is
plotted between the transmittance Vs
log mL (volume) of the standard
solution in each tube. In this way, the
response is duly calculated by
summing up the two transmittances for each level of the test solution.
Finally,
the exact concentration of the calcium
pantothenate in the ‘test sample’
is determined accurately with the aid of the standard concentration-response curve obtained.
(2) Niacin (or Niacinamide)
Preamble. In this particular assay the most
appropriate organism should be such that must be able to fully use up there five
vital and important components, namely : niacin,
nicotinuric acid, miacinamide,
niacinamide, nucleoside, and
coenzymase (an enzyme). This
organism that may criti-cally satisfy the aforesaid requirements happens to be Lactobacillus
plantarum. Interestingly, this acid forming organism is found to be
quite incapable to afford the synthesis of niacin
for its on-going meta-bolic processes. A few other equally important criteria
of this organism are as given under :
·
Non-pathogenic in nature
·
Easy to culture
·
Least affected by various stimulatory or inhibitory
constituents usually present in ‘pharma-ceutical
formulations’ containing niacin.
·
Conveniently grown upon a rather simple stab culture comprising of gelatin, yeast extract, and glucose.
Note. (1) For the assay of niacin, it is cultured
in the assay tubes by actually transferring to the ensuing liquid culture
medium comprising of the basic medium having an optimized quan-tum of added
niacin.
(2) To obtain a measurable response the amount of
niacin present in each tube may range between 0.05 to 0.5 mcg.
(1) Reagents. The various reagents used for the microbial assay of niacin are as enumerated under :
(a) Standard Stock Solution of Niacin (I). It essentially
contains 100 mcg.mL–1 of niacin USPCRS*.
(b) Standard Stock Solution of Niacin (II). It
consists of 10 mcg.mL–1
of niacin USPCRS; and is prepared by
dilution of solution (I) in the ratio 1 : 10, i.e., 1 mL of solution (I) is made up to 10 mL in DW.
(c) Standard Niacin Solution. It
critically contains niacin ranging
between 10-40 ng (i.e.,nanogram). mL–1, and may be
prepared from Solution II by an
appropriate dilution with DW.
(d) Basal Culture Medium Stock Solution. The basal culture medium stock solution may
be prepared by the following requisite proportion of various ingredients and
solutions as enu-merated under :
Casein
hydrolysate solution** : 25 mL
Cystine-tryptophane
solution : 25 mL
Anhydrous
dextrose : 10 g
Anhydrous
sodium acetate : 5 g
Adenine-guanine-uracil
solution : 5 mL
Riboflavin-Thiamine
hydrochloride-Biotin Solution : 5 mL
PABA-Calcium
patothenate-Pyridoxine
hydrochloride
solution : 5 mL
Niacin
solution A : 5 mL
Niacin
solution B : 5 mL
The culture medium is duly perpared by carefully
dissolving anhydrous dextrose and anhydrous sodium acetate into the previously
mixed solutions, and adjusting the pH precisely to 6.8 by the dropwise addition
of 1 M.NaOH. The final volume was made up to 250 mL with DW.
(e) Culture Medium. Into a
series of labeled ‘test tubes’
containing 5 mL of the Basal Culture
Medium Stock Solution [(d) above] 5 mL of water containing
exactly 1 mcg of niacin are incorporated carefully. The
sterilization of all these ‘test tube’
are carried out by first plug-ging each of them with cotton, and subsequently
autoclaving them at 121°C for 15 minutes.
(2) Preparation of Inoculum. Transfer
from the stock culture of Lactobacillus
plantarum cells aseptically into a sterilie test tube containing 10 mL
of culture medium [(e) above]. The resulting culture is duly incubated at a
temperature ranging between 30–37°C for a duration of 16–24 hours. The cell suspension of the said organism is
termed as the inoculum.
(3) Methodology. The various steps that are
involved in the microbial assay of
niacin are de-scribed as under in a sequential manner :
(i) First
and foremost the ‘spectrophotometer’
is duly calibrated according to the proce-dural details mentioned in the ‘official compendia’*.
(ii) Standard Niacin Solution is added
in duplicate into various Standard Niacin Tubes in varying
quantities viz., 0, 0.5, 1.0, 1.5,
2.0, 2.5 ...... 5.0 mL respectively. To each of these tubes add 5.0 mL of the Basal Culture Medium Stock Solution [(d) above] plus sufficient distilled
water to make 10 mL.
(iii) Test Solution Tubes
containing varying amounts of niacin
are carefully prepared by making in duplicate
1, 2, 3, 4, and 5 mL respectively of the ‘test
solution’. To these tubes are added 5 mL of the Basal Culture Medium Stock Solution [(d) above], and followed by water to make upto 10 mL.
(iv) All
the tubes obtained in (iii) above are
duly plugged with cotton, and adequately steri-lized in an ‘autoclave’ (for 15 minutes at 121°C).
(v) After
having brought down the hot tubes to the ambient temperature, they are
carefully inoculated asepticlly with one
drop of inoculum [(2) above], and subsequently be-tween 30–37°C for a
duration of 16 to 24 hours.
(vi) Having
set the percentage transmittance at 1 for the ‘uninoculated blank’, the various transmittance of the inoculated tubes is duly noted, and
recorded.
(4) Calculation : First of
all a ‘Standard Curve’ is plotted
for niacin between :
·
standard transmittances for each level of Standard Niacin Solution, and
·
exact quantum of niacin (in mcg) present
duly in the respective tubes.
Thus,
from the ‘Standard Curve’, one may
easily obtain the niacin precisely
present in the ‘test solution’ of each tube by interpolation.
Finally,
the exact niacin content of the ‘test material’ may be calculated from
the ‘average values’ duly obtained from at least six tubes which should not vary
by more than ± 10% with
respect to the average values.
(3) Vitamin B12 [or Cynocobalamin]
It is
pertinent to state here that the ‘basic
culture medium’ employed for the assay of vitamin B12 is
found to be extremely complex in nature, and essentially comprises of a large
number of varying constituents in
the form of a mixture in solution.
Various
steps are as follows :
(1) First
set of tubes contains solely the measured quantum of a Standard Cyanocobalamin Solution.
(2) Second
set of tubes essentially comprise of the graded volumes of the ‘test sample’ (i.e., unknown).
(3) All
the ‘tubes’ (i.e., first set + second
set) are carefully inoculated with a
small quantity of the culture of Lactobacillus leichmanni, and
subsequently incubated duly.
(4) The
precise extent of growth is assayed by measuring the percentage transmittance
by the help of a standardized (calibrated) spectrophotometer.
(5) The concentration-response curve is now
prepared mediculously by plotting the following two observed parameters :
·
Transmittance values (i.e., response), and
·
Different concentrations (i.e.,dose) of Standard
cyanocobalamin solution.
(6) Ultimately,
the exact quantum of vitamin B12
duly present in the given ‘test sample’
(i.e., unknown) is calculated based on the ‘Standard Curve’ by the interpolation.
4. Assay of Amino Acids
As
discussed earlier the critical and specific requirements of a microorganism for
an ‘amino acid’ may be employed categorically to assay the exact quantum of
the amino acid duly present in a plethora of pharmaceutical formulations or even food products by allowing the particular organism to grow optimally
in a medium containing all the ‘essential
requirements’, and thus the measured doses of the ‘substance’ called be assayed accurately.
Related Topics
