Radioenzymatic [Transferase] Assays
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Microbiological (Microbial) Assays: Antibiotics-Vitamins- Amino Acids
The ‘radioenzymatic assays’ have gained their abundant acceptance and recognition for the assay of aminoglycoside antibiotics.
RADIOENZYMATIC
[TRANSFERASE] ASSAYS
The ‘radioenzymatic assays’ have gained
their abundant acceptance and recognition for the assay of aminoglycoside antibiotics e.g., amikacin, gentamicin,
kanamycin, neomycin, netilmicin, tobramycin, doxorubicin, cephalosporins,
cephamycins, thienamycin, lincomycin, clindamycin, eryth-romycin,
clarithromycin, azithromycin, oleandomycin, spramycins etc ; and chloramphenicol (or Chloromycetine). Importantly, the radioenzymatic assays are exclusively
based upon the fact that the prevailing
inherent microbial resistance to the
said aminoglycoside antibiotics and chloramphenicol is predominantly
associated with the specific as well as the critical presence of certain highly
specialized enzymes* that particularly render the ‘antibiotics’ absolutely inactive
via such biochemical means as : acetylation,
adenylation, and phosphorylation.
It has
been duly proved and established that :
·
aminoglycoside
antibiotics—are susceptible to prominent attack by these
critical and specific enzymes as :
Aminoglycoside acetyltransferases (AAC) ;
Aminoglycoside adenylyltransferases (AAD) ;
Aminoglycoside phosphotransferases (APH).
·
Chloramphenicol—is prone
to predominant attack by the enzyme :
Chloramphenicol
acetyl transferases (CAT).
Mechanism of Action : The
mechanism of action of these enzymes viz,
AAC, AAD, and APH are not the
same :
Acetyltransferases [i.e., AAC]—invariably attack the most susceptible amino moieties (–NH2), and to accomplish this critical function
may require acetyl coenzyme A (AcCoA).
Adenylyltransferases [i.e., AAD] and
Phosphotransferases [i.e., APH]—these enzymes usu-ally attack the
most susceptible hydroxyl moieties
(–OH), and specifically requires adenosine
triphosphate [ATP] i.e., another nucleotide triphosphate.
Applications : As to date quite a few AAC and
AAD enzymes have been judiciously employed
for the radioenzymatic assays.
Example : Both the enzyme and the suitable radiolabelled cofactor [1 – 14C]**
acetyl coenzyme A, or [2 – 3H]***
ATP are used frequently in order to specifically radiolabel the ‘drug substance’ under investigation.
Method— The various steps involved in the assay are as follows :
(1) Enzymes are normally prepared by anyone
of the following two techniques,
(a) Osmotic Shock i.e., by breaking the cells of an appropriate microbial culture by
exposing than to a change of strength of solution therby affording a definite
perceptible alteration in the ‘osmotic
pressure’, and
(b) Ultrasonic Sound-waves i.e., by breaking the cells of a
suitable bacterial culture by means of the high-frequency
ultrasonic sound waves.
Thus, the
said two methods do break open the cells to a considerable extent, and no
purification is required at all.
(2) Radiolabelled drug substance is
subsequently separated from the ensuing reaction mixture soonafter the said reaction has attained completion duly. Thus,
the exact quantum of the extracted radioactivity is observed to be
directly proportional to the exact quantum of the drug substance present in
the given sample.
Note : Separation of two types of antibiotics are accomplished duly as stated under :
(a) Aminoglycoside Antibiotics—by binding them
suitably to phophocellulose paper, and
(b) Chloramphenicol—by making use of an organic
solvent.
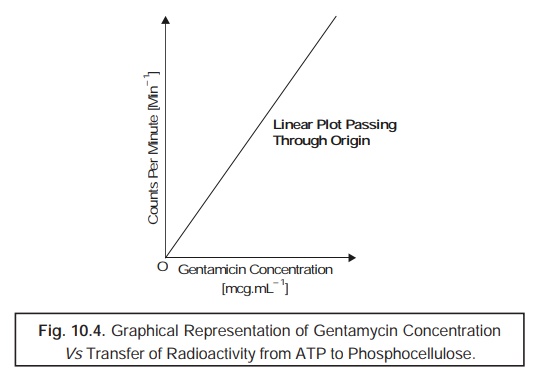
1. Calibration
In a
particular situation when the reactants
are adequately present in enough quantum, and the prevailing reaction attains completion in due
course, one may conveniently plot a graph of the counts per minute (min–1)
Vs concentration of calibrator, which
is found to be linear, as
illustrated in Fig. 10.4.
2. Non-Isotopic Modification
The calibration accomplished by using the radiolabelled drug essentially needs
either a Gei ger Müller Counter or a Scintillation Counter, for measuring
the ensuing radio activity (in mC)
of the ra dioactive chemicals, which being an enormously ex pensive equipment, and a skilled technician. There- fore, in order to circumvent these
glaring untoward se- rious problems one may adopt a photometric varia tion of the
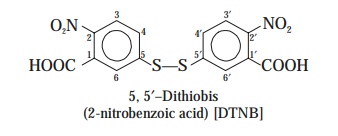
aminoglycoside acetyltransferases [AAC] assay meticulously. For this the sulphydry rea-gent viz., 5, 5′-dithiobis
(2-nitrobenzoic acid) is incorporated carefully into the on-going assay-sys-tem. Thus, the said reagent
specifically interacts with the corresponding coenzyme A (reduced form) duly generated thereby producing a
distinct yellow-coloured product
that may be quantitatively as-sayed by using a previously standardized UV-Visible Spectrophotometer.
(a) Reactions : The two reactions are as follows :
(b) Aminoglycoside
+ Acetyl CoA —→ Acetyl –
Aminoglycoside + CoASH CoASM + DTNB —→ Yellow
Product
Related Topics
