Natural Resistance and Nonspecific Defense Mechanisms [or Defensive Mechanisms of Body]
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Immune Systems
In a broader sense the ensuing interaction existing between a host (human body) and a microorganism designates an excellent unique dynamic phenomenon whereby each and every protagonist critically serves to maximize its overall survival.
NATURAL
RESISTANCE AND NONSPECIFIC DEFENSE MECHANISMS [OR DEFENSIVE MECHANISMS OF BODY]
In a broader
sense the ensuing interaction existing between a host (human body) and a microorganism
designates an excellent unique dynamic phenomenon whereby each and every
protagonist critically serves to
maximize its overall survival. It has been duly observed that in certain
typical instances, after a specific microbe gains its entry or comes in contact
with a host, a distinct positive
mutually beneficial relationship takes place which ultimately becomes integral
to the final health of the host. In
this manner, the microorganisms turn out to be the normal microbiota*. However, in other such cases, the particular
microorganism causes, induces or produces apparent devastating and deleterious
overall effects upon the host ; and,
therefore, may finally even cause death of the host via a dreadful ailment.
Interestingly,
the prevailing environment of a ‘host’
is heavily surrounded with microorganisms, and there lies an ample scope and
opportunity to come in their contact every moment of the day. Nevertheless,
quite a few of these microbes are pathogenic
in nature (i.e., cause disease).
Surprisingly, these pathogens are at times duly guarded and prevented from
producing a disease due to the inherent
competition offered by the normal microbiota. In reality, the invading pathogens are squarely kept away from the host by the ‘normal
microbiota’ by using nutrients, resources, space, and may even yield such
chemical substances which would repel them ultimately.
In
addition to the above stated glaring scientific fact and evidences these ‘normal microbiota’ grossly prevent
colonization of pathogens to a great
extent ; and, thereby, most probably checking the disease (to the host) via ‘bacterial
interference’.
Example : An excellent typical example is
stated as under :
Lactobacilli – present strategically in the female genital tract (FGT) usually
maintain a low pH (acidic), and
thereby exclusively afford the colonization by the pathogenic microbes.
Besides, the corynebacteria located
critically upon the skin surface give rise to the formation of ‘fatty acids’ which ultimately inhibit the phenomenon of
colonization by the pathogenic organisms.
Note : It is an excellent example of ‘amensalism’.
(i.e., symbiosis wherein one
population (or individual) gets affected adversely and the other is
unaffected).
Interestingly,
the ‘normal microbiota’ usually give
rise to protection confined to a certain degree from the invading pathogens ; however, they may themselves turn into pathogenic in character and cause
disease under certain particular circumstances. Thus, these ‘converted pathogens’ are invariably
known as ‘opportunistic
microorganisms’** or pathogens.
Based on
the above statement of facts and critical observations one may conclude that on
one hand pathogen makes use of all
the opportune moments available at its disposal to cause and induct infection,
the host’s body possesses a plethora of ‘defense
mechanisms’ to encounter the infection. In fact, the observed intricacies
prevailed upon by the host-pathogen
relationship are not only numerous but also quite divergent in nature,
which may be classified under the following three heads, such as :
(a) Natural
Resistance,
(b) Internal
Defense Mechanisms, and
(c) Nonspecific
Defense Mechanisms.
The
aforesaid three categories shall now
be discussed separately in the sections that follows :
(1) Natural Resistance
It has
been observed that the two cardinal
aspects, namely : (a) physiological
needs, and (b) meta-bolic
requirements, of a pathogen are an
absolute necessity in establishing precisely the extent vis-a-vis the range of potentially susceptible hosts.
However, the naturally resistant hosts
exert their action in two variant
modes, such as :
·
miserably fail to cater for certain urgently
required environmental factors by
the microbes for their usual growth,
and
·
essentially possess defense mechanisms to resist infection
considerably.
Besides,
there are some other factors pertaining to the host’s general health,
socioeconomic sta-tus, level of nutrition potentiality, and certain intangible
conditions viz., stress, mental
agony, depres-sion etc.
Natural resistance essentially
comprises of the following four
vital and important aspects :
1. Species Resistance
In
general, the fundamental physiologic characteristics of humans, namely : normal body tem-perature may give a
positive clue whether or not a specific
bacterium can be pathogenic in nature.
Likewise, in host-specific e.g., human and bovine species, the tubercle bacillus is found to
cross-infect both humans and cattle having almost an identifical body
temperature.
Salient Features : The salient features of species resistance
are as given under :
(1) inability
of a bacterium to induct disease in
the resistant species under the
natural environ-ments,
(2) critical
production in the specific resistant species of either a localized or a
short-period infection caused solely due to an experimental inoculation vis-a-vis a progressive or gener-alized
ailment in naturally susceptible
species, and
(3) introduction
of experimental disease particularly in the resistant species exclusively
caused by massive doses of the microbes, usually in two different ways :
(a) under
unnatural parameters, and
(b) by an
unnatural route.
2. Racial Resistance
Exhaustive
and intensive studies have amply proved that the very presence of a pathogen in the isolated races give
rise to a gradual selection for
resistant members, because the susceptible
members die of progressive infection ultimately. It may be further
expatiated by the following three glaring examples :
Examples :
(i) Incorporation
of altogether ‘new pathogens’ e.g., tubercle bacillus, by the relatively resist-ant Europeans into an isolated American Indians population*, finally
caused epidemics that almost
destroyed a major proportion of the ensuing population.
(ii) African Blacks (Negros)
invariably demonstrate a relatively high resistance to the tropical diseases, namely
: malaria, yellow fever, and
(iii) Orientals do exhibit a much reduced
susceptibility to syphilis.
3. Individual Resistance
It may be
critically observed that there are certain individuals
who apparently experience fewer or less severe infections in comparison to
other subjects, irrespective of the fact that :
·
both of them essentially possess the same racial
background, and
·
do have the same opportunity for ultimate exposure.
Causation : Individual resistance of this
nature and kind is perhaps on account of :
·
natural in-built resistance factor, and
·
adaptive resistance factor.
Age Factor – is equally important, for
instance :
·
aged
people are more prone to such ailments as :
Pneumonia – most probably due to a possible
decline of the ‘immune functions’
with advancement in growing age.
·
children i.e., very
young individuals are apparently more susceptible to such ‘children’s disease’ as :
Chicken-pox, measles–just prior
to their having acquired enough in-built
resistance/immunity that essentially follows both inapparent and overt contracted infections.
Genetic Factor – Immunodeficiencies** found in some, individuals are caused solely
due to ‘genetic defects’, that
largely enhance the probability and susceptibility to disease.
Other Factors – include malnutrition, personal
hygiene, and an individual’s attitude to sex pro-file ; hazards and nature of
work-environment ; incidence of contacts with infected individuals, and an
individual’s hormonal vis-a-vis endocrine balance – they all do affect the overall frequency as
well as selectivity of some critical ailments.
4. External Defense Mechanisms
In fact,
the external defense mechanisms do
represent another cardinal and prominent factor in natural resistance ; however,
they essentially involve the chemical barriers as well. Besides, two other predominant factors viz., (a) mechanical barriers,
and (b) host secretions, essentially make up the body’s First-Line of Defense Mechanism against
the invading microorganisms.
Mechanical Barriers –
actually comprise of such materials as :
intact (unbroken) skin and mucous
membranes that are practically incapable of getting across to the infectious
agents. However, the said two mechanical barriers viz., intact skin and mucous membranes
do afford a substantial ‘effec-tive
barrier’, whereas hair follicles,
dilatation of sweat glands, or
abrasions do allow the gainful entry
for the microbes into the human body.
Examples : Various typical examples are as
given under :
(1) Large
segment of microbes are duly inhibited by such agents as :
·
low pH (acidity),
·
lactic acid present in sweat, and
·
fatty acids present in sweat.
(2) Mucous secretions caused by respiratory tract (RT), digestive tract
(DT), urogenital tract (UT) plus other such tissues do form an integral
protective covering of the respective mucous
membranes thereby withholding and collecting several microorganisms until they
may be either disposed of effectively or lose their infectivity adequately.
(3) Chemical Substances –
Besides, the ensuing mechanical action caused by mucous, saliva, and tears
in the critical removal of microorganisms, quite a few of these secretions do
con-tain a number of chemical substances
which critically cause inhibition or destruction of microorganisms.
Examples : A few typical examples are as
stated under :
(a) Lysozyme – an enzyme invariably observed
in several body fluids and secretions viz., blood, plasma, urine, saliva,
cerebrospinal fluid, sweat, tears etc., that predominantly do exert an
effective antimicrobial action on account of its inherent ability to lyse some
particular Gram positive microbs by
specifically affording the hydrolysis of peptidoglycan,
(b) Several
other hormones and enzymes are capable of producing
distinct chemical, physi ological, and mechanical effects that may ultimately
cause minimization of susceptibility to reduction, and
(c) The
prevailing inherent acidity or alkalinity of certain ‘body fluids’ possess an apparent deleterious effect upon several
microbes, and helps to check and prevent the potential pathogens for gaining an
easy access to the deeper tissues
present in the body.
(d) Lactoferrin-Lactoferrin is an
iron-containing red-coloured protein found in milk (viz., human and bovine) that essentially possesses
known antibacterial characteristic
features. It is also found in a plethora of body-secretions that specifically and profusely bathe the human mucosal surfaces, namely :
·
bronchial mucous ;
·
seminal fluids ;
·
hepatic bile ;
·
saliva ;
·
nasal discharges ;
·
tears ; and
·
pancreatic juice ;
·
urine.
Lactoferrin forms a vital and important
constituent of the highly particular granules of the ‘polymorphonuclear leukocytes’*.
(5) Transferrin : It
represents the serum counterpart of lactoferrin. In fact, both these typical
proteins essentially possess high
molecular weights ~ 78,000 daltons, besides having several metal-binding critical sites.
Mechanism : Transferrin (as well
as Lactoferrin) critically undergoes ‘chelation’ with the bivalent ferrous iron [Fe2+]
available in the environment, thereby restricting profusely the availability of ferrous ion (i.e., an
essential metal nutrient) to the particular invading microbes.
2. Internal Defense Mechanisms
Internal defense mechanisms emphatically
constitute the ‘second-line of defense’ comprising of the body’s internal mechanisms that
may be critically mobilized against the highly
specific invading bacteria.
Mechanisms : The internal defense mechanisms are of two different types, such as :
(a) Non specific in action – e.g., phagocytosis, and
(b) Specifically aimed at the pathogens – e.g., sensitized cells, and antibodies.
Importantly,
the above two different types are
usually designated as nonspecific
defense mecha-nisms and specific
acquired immunity*.
However,
it is pertinent to state here that while the infection is active the two aforesaid mecha-nisms virtually exert their action simultaneously in order to
rid the body of the so called ‘invading
microbes’. In fact, this very
interrelationship, and the
interrelationships prevailing between the defense mechanisms may be explicitely depicted in Fig. 9.4.
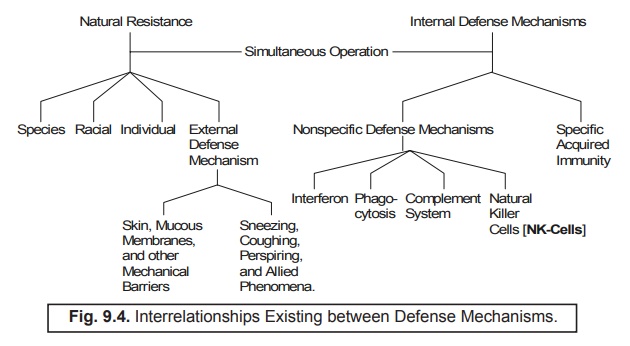
3. Nonspecific Defense Mechanisms
Mother
nature has enabled the ‘human body’
so splendidly as to critically mobilize several factors that act nonspecifically against the possible wide
spread invasion by the ‘foreign organisms’. Interestingly, such cardinal
and vital factors essentially consist of the following four typical examples, namely :
·
complement system,
·
phagocytosis,
·
naturally occurring cytotoxic lymphocytes, and
·
interferon.
Each of
the aforesaid factors shall now be treated individually in the sections that
follows :
1. Complement System
Higher
animal’s serum usually made up of a particular group of ‘eleven proteins’, which are highly specific in nature, and are
widely referred to collectively as the so called complement system by virtue of the fact that its action complements
predominantly to that of some prominent antibody-medi-ated
reactions. In other words, the
complement system critically enacts a pivotal role with respect to the overall generalized resistance
against the infection caused by the ‘pathogens’
; and, therefore, accounts for as the ‘principal
mediator’ of the ensuing specific inflammatory
response.
Mode of Action (Modus
Operandi) : The various steps involved are as follows :
(1) When
the very ‘First Protein’, belonging
to cluster of elevan proteins, gets duly activated there exist distinctly a
prominent ‘sequential cascade’
whereby the ‘active molecules’ duly
come into being via the inactive precursors*.
(2) Some
of the protein variants do get activated very much along the ‘sequential cascade’ that may function
as mediators of a specific response,
and eventually serves as activators
of the next step.
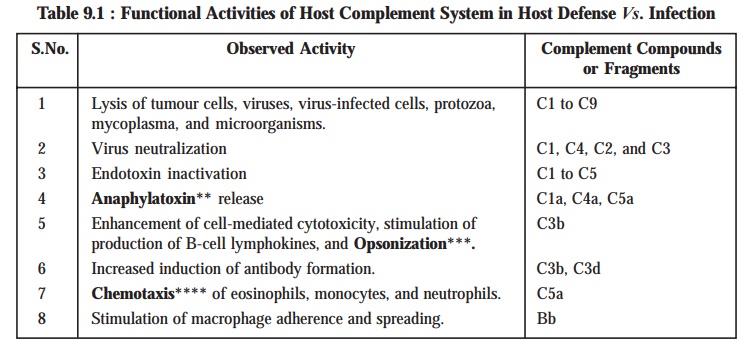
Table 9.1 : Records certain of the functional
activities of the Host Complement
System present duly in the Host Defense against the infection.
Table 9.1 : Functional Activities of Host Complement
System in Host Defense Vs. Infection
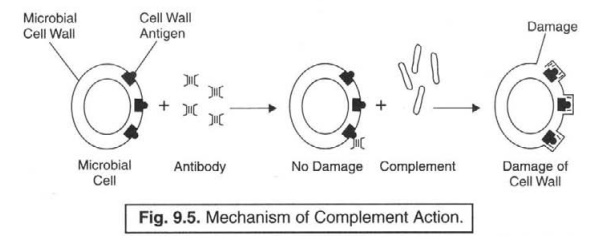
Complement Fixation (or Attachment) : In a
broader perspective, the complement
system is quite capable of attacking and killing the invading cells exclusively after the
antibody gets bound to the cell membrane, thereby specifically initiating the
very phenomenon of complement fixation (or at tachment), which has been
explicitely illustrated in Fig. 9.5.
[Redrawn
From : Vander AJ el al. Human Physiology : The Mechanism of Body Action, McGraw
Hill. New York. 19701
Explanation : Explanation of Fig. 9.5 is as
stated under :
(1) Complement
system do possess many characteristic features.
(2) Recognition
unit present in it predominantly respond to the specific ‘antibody molecules’
which have meticulously identified (recognized) an invading cell.
(3) Receptor
sites do exist which critically combine with the available surface of the
‘foreign cell' on being duly activated.
(4) Activity
of the ‘foreign cell’ should be adequately restricted right in time so as to
reduce the damage eventually caused to the host’s own cells.
(5) The
resulting accomplished ‘limitation’ is actually brought about proportionately
by the help of two distinct functionalities, such as :
(a) spontaneous
decay of activated complement, and
(b) interference
afforded by inhibitors and destructive enzymes.
Mechanisms
of Complement Action in Microbial Lysis : The eleven components duly present in
a complement are named as per the following rules and guidelines, namely :
(1) Each
and every component has been assigned a particular number strictly according to
its discovery, and that number is usually preceded by the below letter ‘C’.
(2) Surprisingly,
the very first four components fail to interact in the desired order of their
discovery, but instead of the sequence Cl, C4, C2 and C3.
(3) The
remainder of the components certainly and strictly react in the suitable
numerical or¬der viz., C5, C6, C7, C8, and C9.
(4) However,
Cl essentially comprise of three subcomponents viz., Clq, Clr, and Cis.
(5) Fragments
of components, obtained as a consequence of cleavage by other components,
acting invariably as enzymes are adequately assigned the lowercase letters a,
b, c, d or e such as : C3a and C3b.
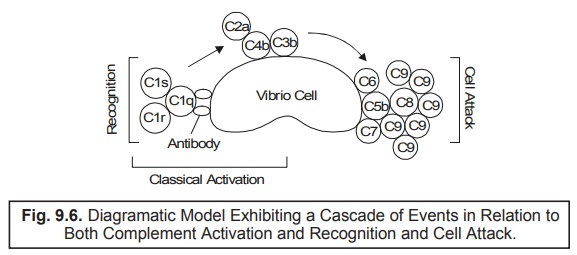
In fact,
one may vividly expatiate the underlying mechanisms of component action in
microbial lysis as depicted in Fig. 9.5, in a more elaborated fashion, as
illustrated in Fig. 9.6 thereby exhibiting a cascade of events in relation to
both complement activation and recognition, ultimately culminating in cell attack. Summararily, it
represents as the classical or antibody-dependent pathway that
prevalently need to be activated by
specific antibody : C1, C4, C2 and C3.
[Adapted
From : Pelczar MJ et al. : Microbiology, Tata McGraw Hill
Publishing Co., LTD., New Delhi, 5th edn., 1993]
2. Phagocytosis
Phygocytosis may be defined as — ‘the engulfing of microorganisms or other
cells and for-eign particles by phagocytes’.
Alternatively,
phagocytosis (from the Greek words
for eat and cell) referts to — ‘the
phenom-enon of ingestion of a microorganism or any particulate matter by a
cell’.
Interestingly,
the human cells which critically
carry out this ardent function are collectively known as phagocytes, such as : all types
of WBCs, and derivatives of WBCs.
Actions of Phagocytic Cells : In this
event of a contracted infection, both
monocytes* and granulocytes** usually
get migrated to the infected area. Interestingly, during this process of
migration, the monocytes do get enlarged to such a dimension and size that they
finally develop into the actively phagocytic
macrophages.
Types of Macrophages : There
are, in fact, two major categories of
the macrophages, such as :
(a) Wandering Macrophages : Based on
the glaring fact that these cells (monocytes)
do have a tendency to leave the blood and subsequently migrate via the tissue cells to the desired
infected areas, they are commonly known as wandering
macrophages.
(b) Fixed Macrophages (or Histocytes) : A monocyte that has eventually become a resident in tissue. Fixed macrophages or histocytes are invariably located in
certain specific tissues and organs of the body. In fact, they are found
abundantly in various parts of a human body, for instance :
·
Bronchial tubes ;
·
Lungs (alveolar macrophages) ;
·
Bone marrow ;
·
Nervous system (microglial cells ) ;
·
Lymph nodes ;
·
Peritoneal cavity (surrounding abdominal organs) ;
·
Liver (Kupffer’s cells ) ;
·
Spleen ;
Importantly,
the macrophage variants critically
present in the body strategically constitute the mononuclear phagocytic (reticuloendothelial) system.
2.1. Functions of Phagocytes (or Phagocytic Cells) :
It has
been duly observed that when an
infection gets contracted one may apparently observe a distinct shift taking
place predominantly in the particular types of WBC which runs across the blood
stream. Thus, the following cardinal points may be noted, carefully :
Granulocytes – particularly the ‘neutrophils’ occur overwhelmingly in
the initial phase of infection, at
this point in time they are found to be extremely phagocytic in nature.
Distinct
aforesaid dominance is evidently shown by the presence of their actual number
in a differential WBC count.
With the
progress of contracted infection, the macrophages also predominate – scavenge –
phagocytize remaining live/dead/dying microorganisms.
Enhanced
number of monocytes, that eventually develop into the corresponding
macrophages, is adequately reflected in the WBC-differential count explicitely.
Blood and
lymph containing bacteria when made to pass via
various organs in the body having fixed
macrophages, cells of the mononuclear
phagocytic system ultimately get rid of the bacteria by phagocytosis.
Mononuclear
phagocytic system also helps in the critical disposal of the worn-out blood cells.
Table 9.2
records the classification as well as a summary of phagocytic cells and their
functions.
Table : 9.2 : Classification and Functions of
Phagocytes
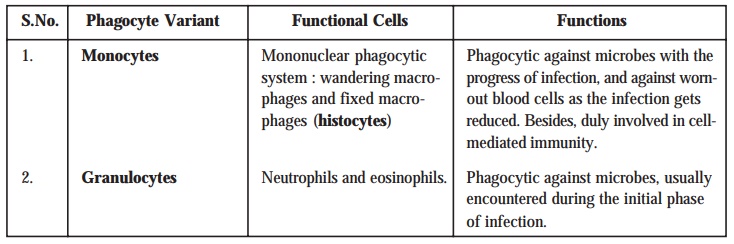
2.2. Mechanism of Phagocytosis :
In order
to understand the exact and precise
mechanism of phagocytosis, we may have to divide the phenomenon of phagocytosis, as illustrated in Fig. 9.6, into four cardinal phases, such as : chemotaxis, adherence, ingestion, and digestion. These four
dis-tinct phases shall now be treated briefly in the sections that follows from
[A] through [D] :
[A] Chemotaxis [Syn : Chemotropism] :
Chemotaxis may be defined as — ‘the movement of additional white blood
cells to an area of inflammation in response to the release of chemical
mediators by neutrophils, monocytes, and injured tissue’.
In other
words, chemotaxis refers to the
chemical attraction of the phagocytes to microbes.
Importantly,
the various ‘chemotactic chemical
susbtances’ which specifically attract the phagocytes happen to be such
microbial products as components of :
·
white blood cells (WBCs),
·
damaged tissue cells, and
·
peptides derived from complement.
[B] Adherence :
Adherence refers to the act or condition of
sticking to something. In fact, it represents the ensu-ing adherence of
antigen-antibody complexes or cells coated with antibody or complement to cells
bearing complement receptors or Fe receptors. It is indeed a sensitive detector
of complement-fixing antibody.
Because, adherence is intimately related to phagocytosis, it represents the
attachment of the later’s plasma membrane onto the critical surface of the
bacterium or such other foreign material. Nevertheless, adherence may be hampered by the specific presence of relatively larger capsules or M protein*. Besides, in certain instances adherence takes place quite easily and conveniently, and the microbe gets phagocytized rapidly.
[Adapted
From : Tortora et al : Microbiology : An Introduction, The
Benjamin/Cummings Pub-lishing Co., Inc., New York, 5th edn., 1995]
[C] Ingestion :
In usual
practice adherence is followed by ingestion. One may vividly notice that
during the phenomenon of ingestion, the plasma membrane belonging to the phagocyte gets extended in the form of
distinct projections usually termed
as pseudopods which eventually
engulf the bacterium. Thus, once the bacterium gets duly surrounded, the pseudopods meet and fuse ultimately,
thereby surround-ing the bacterium with a particular ‘Sac’ known as phagocytic
vesicle or phagosome.
[D] Digestion :
Digestion refers to the particular phase of phagocytosis, wherein the respective phagosome* gets detached from the
plasma membrane and duly enters the cytoplasm. Later on, within the cytoplasm
the phagosome meticulously gets in
touch with the lysosomes** which
essentially comprise of two important
components, namely :
·
digestive enzymes, and
·
bactericidal substances.
Modus
Operandi [or Mode
of Action] : The
various steps involved are as given below :
(1) Both phagosome and lysosome membranes upon contacting each other invariably gets fused
to result into the formation of a ‘single
larger structure’ termed as ‘phagolysosome’.
(2) Interestingly,
the integral contents of the phagolysosome
usually ‘kills’ most types of
microorganisms within a span of 10–30 minutes. The most plausible and possible
reason for such a marked and pronounced bactericidal
effect is perhaps due to the specific
contents of the lysosomes.
(3) Residual body : After
completion of the process of digestion the actual contents of the phagolysosome are duly brought into
the cell by ‘ingestion’ ; and,
therefore the phagolysosome essentially
and exclusively comprises of the indigestible material, which is usually known as the ‘residual body’.
(4) Residual body subsequently
takes a step forward toward the cell boundary and critically discharges its ‘waste products’ very much outside the cell.
A Few Exceptions : These
exceptions are as stated below :
(a) Toxins
of certain microorganisms viz.,
toxin-producing Staphylococci plus the bacterium Actinobacillus (present
in dental plaque, may actually exert a cidal effect upon the phagocytes.
(b) Some
other microbes, for instance : Chlamydia, Leishmania, Mycobacterium,
and Shigella
together with the ‘malarial parasites’
may possibly dodge and evade the various compo-nents of the immune system by
gaining an access into the phagocytes.
(c) Besides,
the said microorganisms may virtually block the ultimate fusion between phagosome and lysosome, as well as the adequate process of acidification (with
HCl) of the digestive enzymes.
3.3. Natural Killer Cells [NK Cells]
It has
been amply proved and widely accepted that the body’s cell-mediated defense system usually makes use of such cells that
are not essentially the T cells***.
Further, certain lymphocytes that are known as natural killer (NK) cells, are quite capable of causing destruction
to other cells, particu-larly (a) tumour cells, and (b) virus-infected cells.
However, the NK cells fail to be
immunologically specific i.e., they
need not be stimulated by an antigen.
Nevertheless, the NK cells are not found to be phagocytic in nature, but should
definitely get in touch (contact) with the target
cell to afford a lysing effect.
3.4 Interferons [IFNs]
Issacs
and Lindenmann (1957) at the National Institute of Medical Research, London
(UK) discovered pioneerly the interferons
(IFNs) while doing an intensive study on the various mechanisms associated
with the ‘viral interference’.
It is,
however, an established analogy that viruses exclusively depend on their
respective host cells to actually cater for several functions related to viral multiplication ; and, therefore,
it is almost difficult to inhibit completely viral multiplication without affecting the host cell itself simultaneously.
Importantly, interferons [IFNs] do
handle squarely the ensuing infested
host viral infections.
Interferons [IFNs] designate — ‘a particular class of alike antiviral
proteins duly generated by some animal cells after viral stimulation’.
It is,
therefore, pertinent to state here that the critical interference caused
specifically with viral multiplication is the prime and most predominant role
played by the interferons.
3.4.1. Salient Features : The salient features of interferons may be summarized as
stated under :
(1) Interferons are found to be exclusively host-cell-specific but not virus-specific.
(2) Interferon of a particular species is active
against a plethora of different viruses.
(3) Not
only do various animal species generate interferon
variants, but also altogether various kinds of cells in an animal give rise
to interferon variants.
(4) All interferons [IFNs] are invariably small proteins having their molecular
weights ranging between 15,000 to 30,000. They are observed to be fairly stable
at low pH range (acidic), and are quite resistant to heat (thermostable).
(5) Interferons are usually produced by virus-infected host cells exclusively
in very small quantum.
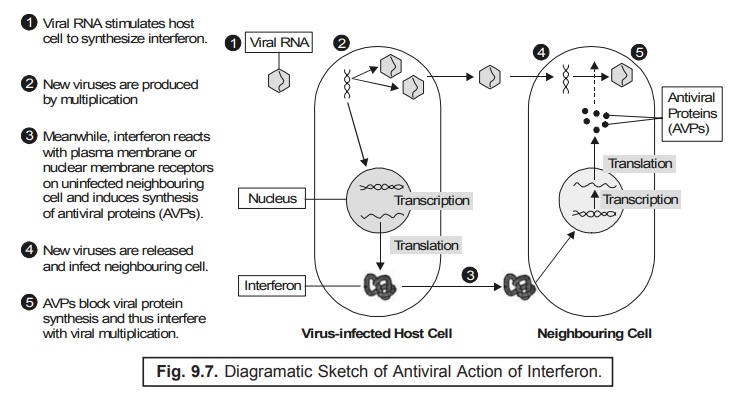
(6) Interferon gets diffused into the uninfected
neighbouring cells as illustrated in Fig. 9.7.
Explanation : The various steps involved are as
follows :
(1) Interferon happens to interact with plasma or nuclear membrane receptors, including the uninfected cells to produce largely mRNA essentially required for the critical synthesis of antiviral proteins (AVPs).
(2) In
fact, AVPs are enzymes which causes
specific disruption in the different
stages of viral multiplication.
Examples : These are as given under :
(a) One
particular AVP inducts the
inhibition of ‘translation’ of viral mRNA by affording complete
blockade in the initiation of the ensuing protein
synthesis,
(b) Another
AVP causes the inhibition of the
phenomenon of ‘polypeptide elongation’,
and
(c) Still
another AVP takes care of the
process of destruction with regard to mRNA
before
‘translation’.
3.4.2. Interferon : An Ideal Antiviral Substance : Various
cardinal points are as stated below
:
·
Prevailing ‘low
concentrations’ at which interferon
affords inhibition of viral
multiplica-tion are found to be absolutely nontoxic to the uninfected cells.
·
Interferon
possesses
essentially a good number of beneficial characteristic properties.
·
Interferon
is
distinguishably effective for only short span.
·
Interferon
plays a
pivotal and vital role in such critical infections which happen to be quite acute and transient in nature, for instance : influenza and common colds.
Drawback : Interferon has a
serious drawback, as it has practically little effect upon the viral multiplication in cells that are
already infected.
3.4.3. Interferon Based on Recombinant DNA
Technology : In the recent past ‘interferon’ has acquired an enormous recognition and importance
by virtue of its potential as an antineoplastic
agent, and, therefore, enabled its
production in a commercial scale globally on a top public-health priority. Obviously, the interferons specifically produced by means of the recombinant DNA technology are usually
termed as recombinant interferons
[rINFs]. The rINFs have gained
an overwhelming global acceptability,
popularity, and utility due to two extremely important reasons, namely
: (a) high purity, and (b)
abundant availability.
Usefulness of rINFs : Since
1981, several usefulness of rINFs have
been duly demonstrated and observed,
such as :
Antineoplastic activity – Large
dosage regimens of rINFs may exhibit
not so appreciable overall effects
against certain typical neoplasms (tumours), whereas absolute negative effect
on others.
However,
the scanty results based on the exhaustive clinical trials with regard to the
usage of rINFs towards anticancer
profile may be justifiably attributed to the following factual observations, such as :
·
several variants of interferons vis-a-vis definitive antineoplastic
properties,
·
rINFs in
cojunction with other known
chemotherapeutic agents might possibly enhance the overall antineoplastic activity,
·
quite significant and encouraging results are duly
achievable by making use of a combina-tion of :
rINFs + doxorubicin*
or rINFs +
cimetidine**
·
subjects who actually failed to respond reasonably
well earlier to either particular
chemo-therapy or follow up treatment
with interferon distinctly showed remarkable improve-ment when again
resorted to the ‘original chemotherapy’.
3.4.4. Classical Recombinant Interferons [rIFNs] : There are
quite a few classical recombinant
interferons [rIFNs] have been meticulously designed and screened
pharmacologically to establish their
enormous usefulness in the therapeutic armamentarium. A few such rIFNs shall now be treated briefly in
the sections that follows :
[A] Interferon-α [Syn : Alfa-interferon ; Leukocyte interferon ; Lymphoblastoid interferon ;]
Interferon-α is a
glycopeptide produced by a genetic engineering techniques based on the human sequence. It does affect several
stages of viral infections, but primarily inhibits the viral-protein trans-lation.
It is
invariably employed to prevent and combat the hepatitis B and C infections. In usual practice the drug is
administered either via subcutaneous
(SC) route or intramuscular (IM) route. However, it gets rapidly inactivated
but generally the overall effects outlast the ensuing plasma concentration.
Toxicities – include neurotoxicity, flu-like
syndrome, and bone-marrow suppression.
Drug interactions – may
ultimately result from its ability to minimize the specific hepatic syn-drome P450-mediated metabolism.
Interferon Alfa-2A, Recombinant [Syn : IFA-α A ;
R0-22-8181 ; Canferon ; Laroferon ; Roferon-A ;]
Interferon alfa-2A refers to
the recombinant HuIFN-α produced in E.
coli, and made up of 165 amino acids.
Characteristic Pharmacologic Activities : These are
as follows :
(1) Enhances
class I histocompatibility molecules
strategically located on lymphocytes.
(2) Increases
the production of ILs-1 and -2 that critically mediates most of the therapeutic and toxic effects.
(3) Regulates
precisely the antibody responses.
(4) Increases
NK cell activities.
(5) Particularly
inhibits the neoplasm-cell growth via its distinct ability to inhibit
appreciably the protein synthesis.
(6) Being
antiproliferative in nature it may exert
its immunosuppressive activity.
(7) Action
on the NK cells happens to be the
most vital for its antineoplastic
action.
(8) Approved
for use in hairy-cell leukemia and AIDS-related Kaposi’s sarcoma.
(9) Drug
of first choice for the treatment of renal-cell
carcinoma.
(10) Preliminary
clinical trials ascertained virtually its promising efficacy against quite a
few typical disease conditions as : ovarian
carcinoma, non-Hodgkin’s lymphoma, and meta-static carcinoid tumour.
(11) Besides,
it exhibits marked and pronouned antiviral
activity against the RNA viruses.
(12) Effective
in the treatment of varicella in immunocompromised children, non-A and non-B
hepatitis, genital warts, rhinoviral colds, possible opportunistic bacterial infections in renal and transplant recipients.
(13) Increases
the targetting process associated
with monoclonal antibody (MAB)-tethered
cytotoxic drugs to the neoplasm cells.
[c] Interferon Alfa-2B, Recombinant [Syn
: IFNα2 ; Introna; Intron A ; Viraferon ; Seh-30500 ; YM-14090
;] ;
The recombinant HuIFNα is
produced in E. coli. Therapeutic Applications : are as
stated under :
(1) Approved
for use in several disease conditions as : hairy-cell
leukemia, AIDS-related Kaposi’s
sarcoma, myclogenous leukemia, melanoma, chronic hepatitis, and condylomata
acuminata.
(2) Most
of its actions are very much similar to those of rIFN-αA.
Related Topics
