Centrally Acting Muscle Relaxants
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Skeletal Muscle Relaxants
a. Carbamates i. Meprobamate b. Glycerol monoethers and analogues (i) Mephenesin ii. Guaifenesin iii. Methocarbamol (Robinax) iv. Chlorphenesin Carbamate (Maolate) c. Substituted alkanediols i. Metaxalone (Skelaxin) ii. Lorbamate iii. Xilobam d. Benzoxazole analogues i. Chlorzoxazone (New Panazox, Ontacplus) e. Heterocyclic bases i. Fletazepam ii. Nefopam f. Imidazoline analogues i. Dantrolene (Dantrium) g. Miscellaneous i. Cyclobenzaprine HCl ii. Baclofen (Liofen, Lioresal) iii. Orphenadrine Hydrochloride
SYNTHESIS AND DRUG PROFILE
Centrally Acting Muscle Relaxants
General mode of action: These selectively depress spinal and supra-spinal polysynaptic reflexes involved in the regulation of muscle tone without significantly affecting mono synaptically mediated stretch reflex.
a. Carbamates
i. Meprobamate
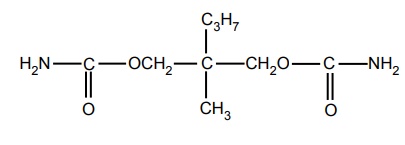
Synthesis and drug profile is discussed under Sedatives and Hypnotics sec III
b. Glycerol monoethers and analogues
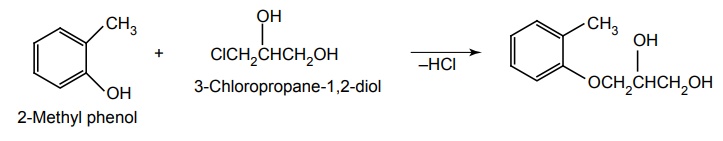
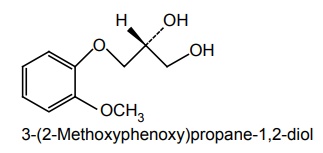
Synthesis of Mephenesin (Tolserol) and Guaifenesin
(i) Mephenesin
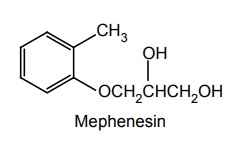
Synthesis
Metabolism of mephenesin derivatives: The major metabolitc product of mephenesin is β-(o-toloxy)lactic acid, which is inactive. Because of easy metabolism (oxidation) of mephenesin to the corresponding lactic acid, its carbamate or succinate derivatives show longer duration, although less active than the parent drug.
Dose: Mephenesin: Usual oral dose is 0.5 or 1g for 1 to 6 times per day as per requirement.
ii. Guaifenesin
Properties and uses: It is a white or almost white crystalline powder, soluble in alcohol, sparingly soluble in water and used as an expectorant.
Assay: The substance is dissolved in freshly prepared mixture of 1 volume of acetic anhydride and 7 volumes of pyridine boil under a reflux condenser and then cooled. To this add water and titrate with 1 M sodium hydroxide using phenolphthalein as indicator. End point is the appearance of pink colour.
iii. Methocarbamol (Robinax)

Synthesis
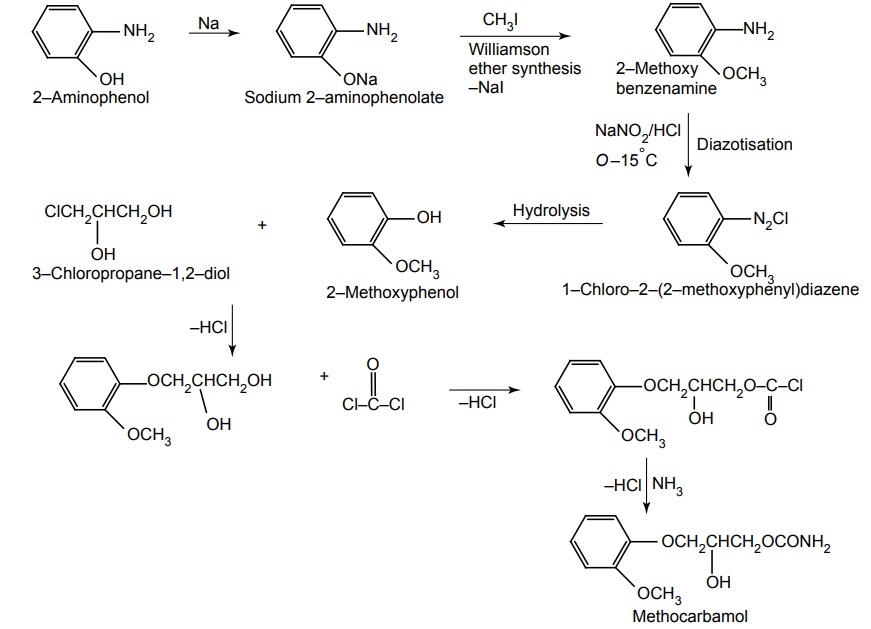
Uses: Used in Parkinsonism and also in cerebro-vascular mishaps.
Dose: Initial oral dose is 1.5 to 2 g, 4 times daily for the first 2 or 3 days followed by 2.25 to 4.5 g per day in 2 or 4 divided doses; I.V., 1 to 3 g per day administered at a rate not exceeding 0.3 g/min; by I.M., 1 g every 8 h.
iv. Chlorphenesin Carbamate (Maolate)
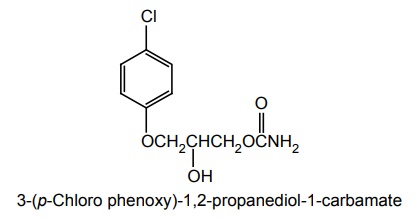
Synthesis
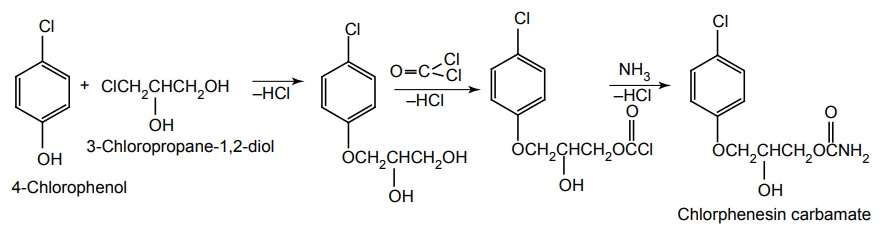
Uses: It is used to diminish skeletal muscle spasms resulting from osteoarthritis and vertebral disk syndrome.
Dose: The initial usual dose is 800 mg thrice/day and reduced to 400 mg four times/day, or less as required.
c. Substituted alkanediols
i. Metaxalone (Skelaxin)
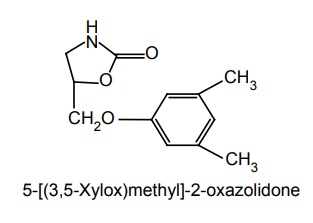
Synthesis
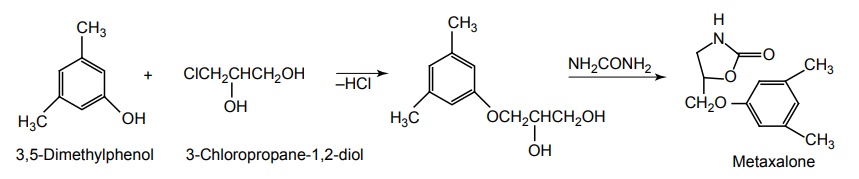
Uses: It is used for local skeletal muscle spasms.
Dose: The usual dose is 800 mg 3 or 4 times/day.
ii. Lorbamate
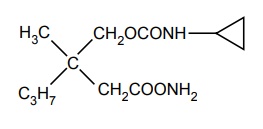
Synthesis
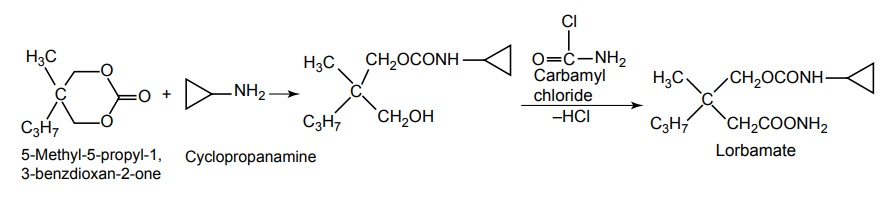
Uses: Used as a muscle relaxant.
iii. Xilobam
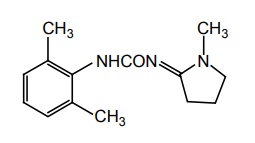
Synthesis
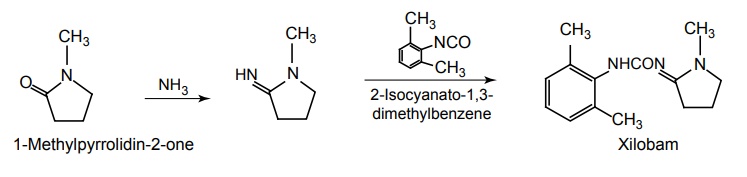
d. Benzoxazole analogues
i. Chlorzoxazone (New Panazox, Ontacplus)
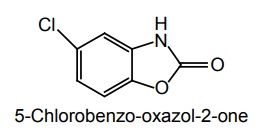
Synthesis
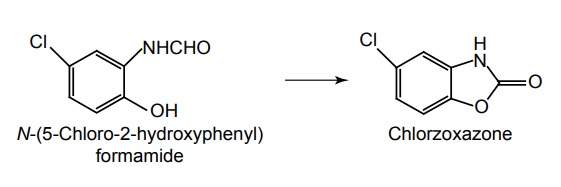
Uses: It is obtained by cyclization of the o-hydroxyl benzformamide. Used as skeletal muscle relaxant for the reduction of painful muscle spasm, such as mycositis sprains and acute or chronic back pain.
Dose: The usual initial dose is 500 mg 3 or 4 times/day, maintenance dose is 250 mg.
e. Heterocyclic bases
Mode of action of benzodiazepines: These drugs produce centrally mediated skeletal muscle relaxation by acting on the benzodiazepine receptors in midbrain reticular formation and on the limbic system. It acts on the benzodiazepine receptors, increases the chloride influx, and produces hyperpolarization and decreases the firing rate of neurons and inhibits the calcium dependent release of neurotransmitters.
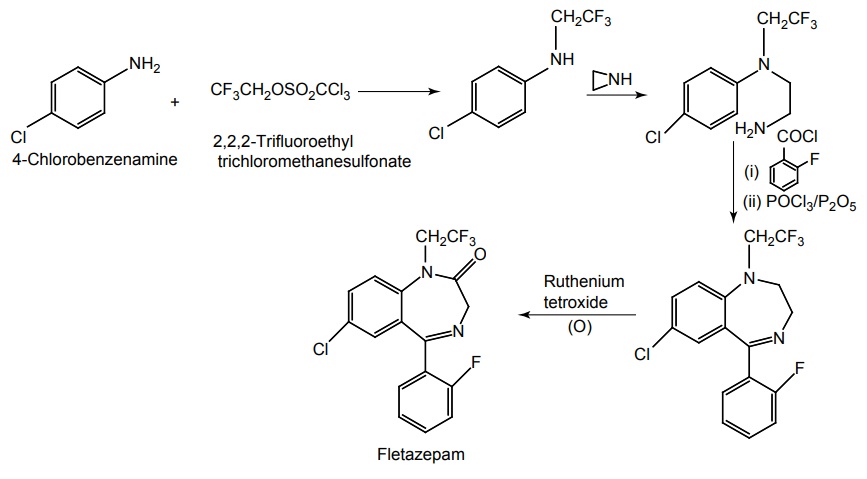
i. Fletazepam
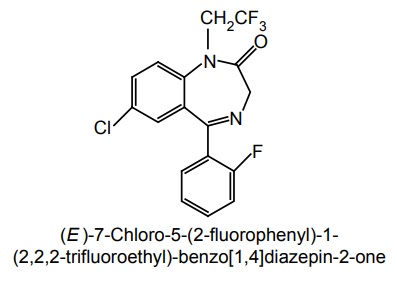
Synthesis
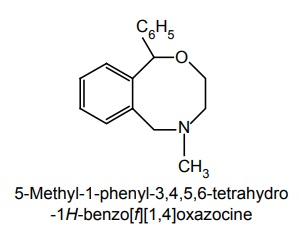
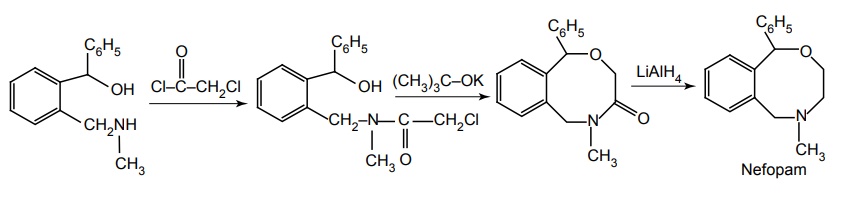
ii. Nefopam
Synthesis
f. Imidazoline analogues
i. Dantrolene (Dantrium)
Mode of action: This does not affect the neuromuscular transmission of membrane action potential, but uncouples contraction from depolarization of muscle membrane. Depolarization triggered release of calcium from sarcoplasmic reticulum is reduced and the contraction of muscle is abolished.
Dose: The initial dose by oral route is 25 mg/day, slowly increased over a period of 7 weeks to 100 mg, 3 to 4 times/day.
Synthesis
Step I. Synthesis of 5-(4-nitrophenyl)-2-furanal
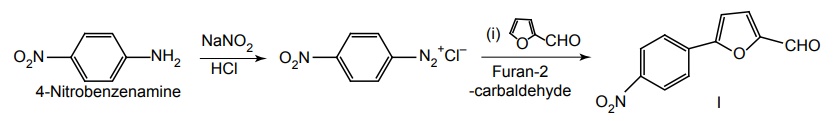
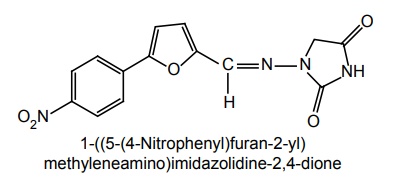
Step II. Synthesis of 4-Amino imidazolidin-2,4-dione
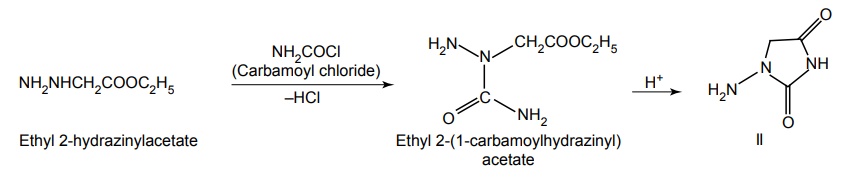
Step III. Condensation of product of Step I and II
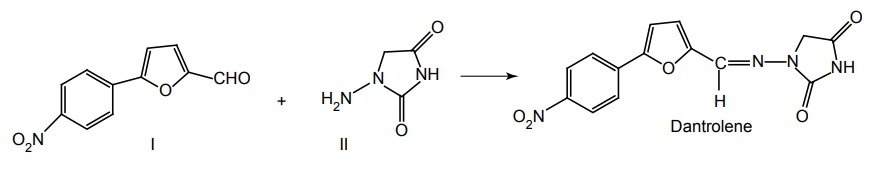
Metabolism: It is slowly metabolized to give 5-hydroxy and acetamide metabolites.
Uses: Used for the control of the spasticity, resulting from spinal cord injury, stroke, and multiple sclerosis.
g. Miscellaneous
i. Cyclobenzaprine HCl
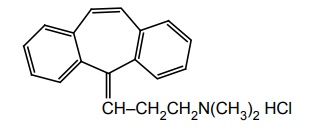
Synthesis
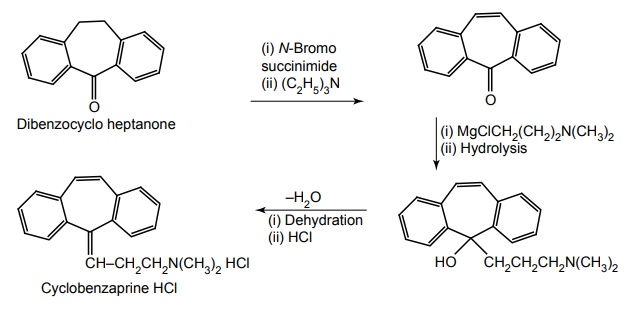
Uses: It is a centrally acting skeletal muscle relaxant that relieves acute and painful muscle spasm.
ii. Baclofen (Liofen, Lioresal)
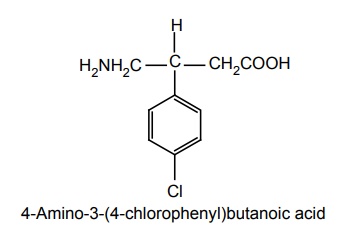
Synthesis
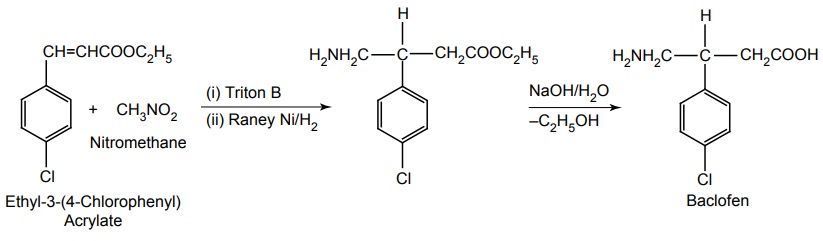
Uses: Useful in treating spasticity associated with spinal cord tensions, as in multiple sclerosis.
Dose: The initial dose is 5 mg thrice/day increased by 15 mg/day every fourth day to 20 mg thrice/day.
iii. Orphenadrine Hydrochloride
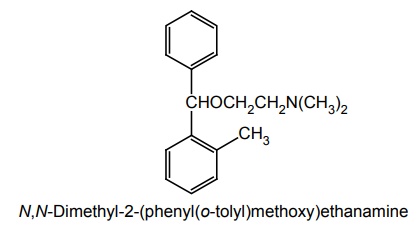
Synthesis
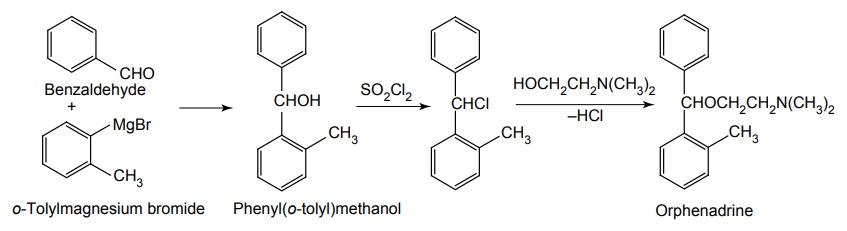
Uses: Used in the control of the symptoms of Parkinson’s disease.
Related Topics
