Drugs affecting brain dopamine
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antiparkinsonism Agents
Antiparkinsonism Agents: Antiparkinsonism Agents - Synthesis and Drug Profile - a. Levodopa (Bidopal, Benspar, Madopar) b. Carbidopa (Lodosyn) c. Amantadine HCl (Symmetrel) d. Bromocryptine mesylate (Parlodel, Proctinal, Sicriptin) - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile
SYNTHESIS AND DRUG PROFILE
Drugs affecting brain dopamine
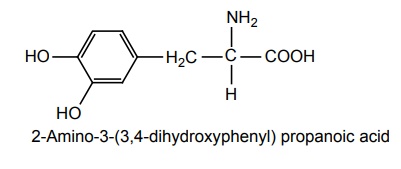
a. Levodopa (Bidopal, Benspar, Madopar)
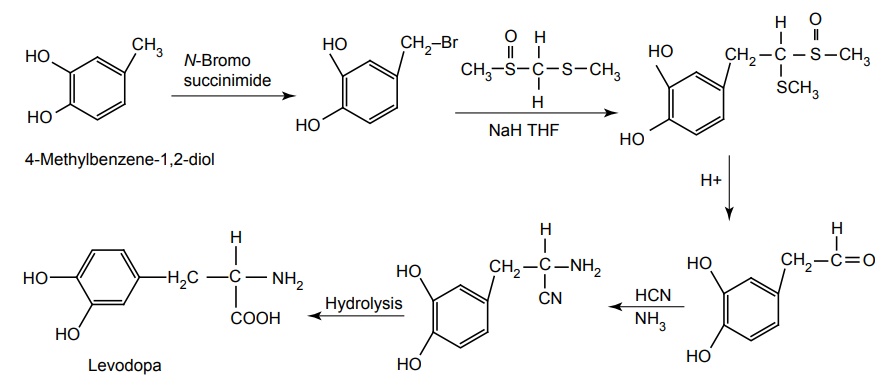
Synthesis
Route-I. From: 4-Methylbenzene-1,2-diol
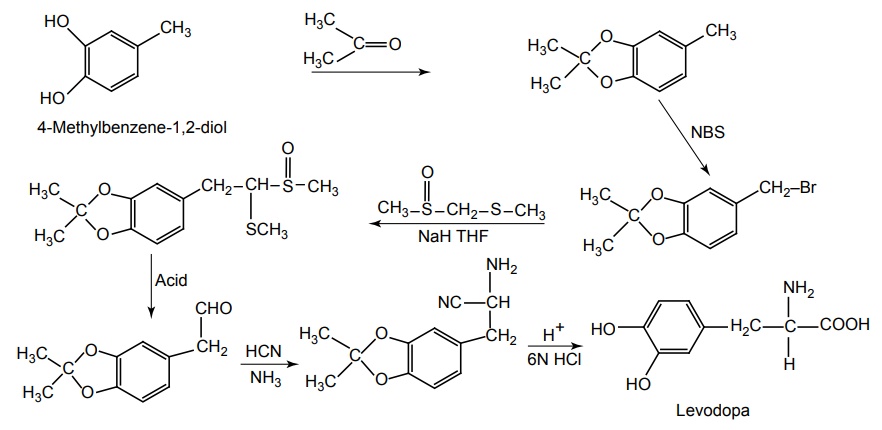
Route-II. From: 4-Methylbenzene-1,2-diol
Metabolism: Levodopa is rapidly absorbed by active transport in gastro intestinal (GI) tract and once absorbed, 95% is decarboxylated in the periphery to the dopamine, which is further metabolized to dihydrophenylacetic acid and homovanillic acid, which are excreted through urine.
Properties and uses: It is a white or almost white crystalline powder, freely soluble in 1 M hydrochloric acid, slightly soluble in water and insoluble in ethanol. Used as dopamine precursor and in the treatment of Parkinson’s disease. It is also used to treat the parkinsonism-like neurological syndrome of manganese intoxication, in which there is also a deficiency of dopamine in the basal ganglia. Levodopa is a precursor of melanin and may activate latent malignant melanoma. The effects of levodopa on bradykinesia and rigidity are more rapid and complete than the effects on tremor.
Assay: Dissolve the sample in anhydrous formic acid by heating, if necessary. To this add anhydrous acetic acid, dioxan, and crystal violet and titrate with 0.1 M perchloric acid. End point is the appearance of green colour.
Storage: It should be stored in well-closed airtight containers and protected from light.
Dose: The initial oral dose is 100 mg to 1 g/day in divided doses with meals; maintenance dose is 2.5 to 6 g daily and must not exceed 8 g.
Dosage forms: Levodopa capsules I.P., B.P., Levodopa tablets I.P., B.P., Co-beneldopa capsules B.P., Dispersible co-beneldopa tablets B.P., Co-careldopa tablets B.P.
b. Carbidopa (Lodosyn)
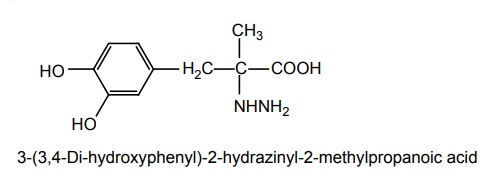
Synthesis
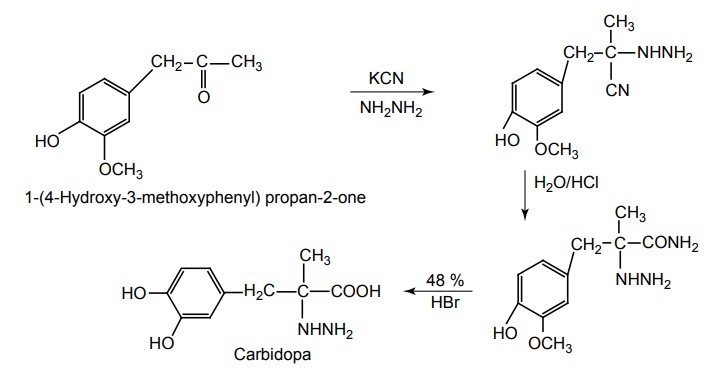
Metabolism of Carbidopa: It is a synthetic amino acid precursor of (–) norepinephrine, which is absorbed from the gut and metabolized to norepinephrine. It is further metabolized by COMT and MAO to form metanephrine and normetanephrine. Further they undergo for the metabolism by MAO to 3-methoxy-4hydroxy mandilic acid.
Properties and uses: It is a white or yellowish-white powder, dissolves in dilute mineral acids, slightly soluble in water, sparingly soluble in alcohol, insoluble in methylene chloride. It has no direct therapeutic actions on its own, but rather is used only to protect levodopa and L-5-hydroxytryptophan. Both of which are decarboxylated by aromatic amino acid decarboxylase. When it is given concomitantly with levodopa, only about 25% as much levodopa is needed. The onset of response is more rapid.
Assay: Dissolve the substance in anhydrous acetic acid and titrate with 0.1 M Perchloric acid. Determine the end point potentiometrically
Storage: It should be stored in well-closed airtight containers and protected from light.
Dosage forms: Co-carbidopa tablets I.P., B.P.
c. Amantadine HCl (Symmetrel)
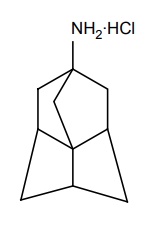
Mode of action: Amantadine appears to act by promoting presynaptic synthesis and release of dopamine in the brain. It acts on glutamate receptors through which dopaminergic system exerts the possible influence on regulating the D1 and D2 receptors.
Properties and uses: It is a white or almost white crystalline powder, and it sublimes when heated. Freely soluble in water and in alcohol. Amantadine an organic cage amine, is an antiviral agent useful in preventing and treating influenza A2. It possesses both antiparkinsonism and antiviral activity.
Assay: Dissolve the sample in a mixture of 0.01 M Hydrochloric acid. Perform potentiometric titration, using 0.1 M sodium hydroxide.
Dose: In the treatment of Parkinsonism initial dose is 100 mg/day, increased to 100 mg twice daily, after one week.
Dosage forms: Amantadine HCl capsules I.P., Amantadine capsules B.P., Amantadine oral solution B.P.
d. Bromocryptine mesylate (Parlodel, Proctinal, Sicriptin)
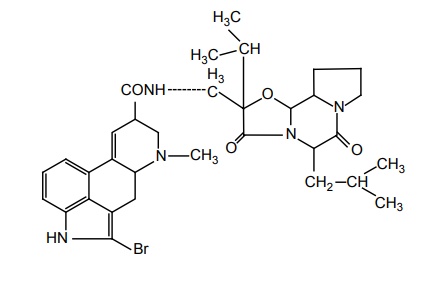
Properties and uses: It is a white or slightly coloured fine crystalline powder, insoluble in water, soluble in methanol and ethanol, sparingly soluble in methylene chloride. It is an ergot derivative. Bromocriptine mimics the action of dopamine. Addition of a bromine atom renders the alkaloid criptine a potent dopaminergic agonist at D2 receptor and an antagonist at D1 sites. It is used as an adjunct to levodopa.
Assay: Dissolve the sample in a mixture of 10 volumes of anhydrous acetic acid and 70 volumes of acetic anhydride. Titrate with 0.1 M perchloric acid and determine the end point potentiometrically.
Storage: Bromocriptine is very sensitive to light, and hence, it should be stored in well-closed airtight container, protected from light.
Dosage forms: Bromocriptine mesylate tablets I.P., Bromocriptine mesylate capsules I.P., Bromocriptine capsules B.P., Bromocriptine tablets B.P.
Related Topics
