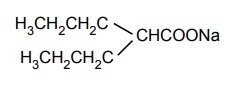
Sodium Valproate
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Anticonvulsants
Anticonvulsants : Sodium Valproate (Epilex, Epival, Valparin) - Synthesis and Drug Profile - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile
SYNTHESIS AND DRUG PROFILE
Miscellaneous
Sodium Valproate (Epilex, Epival, Valparin)
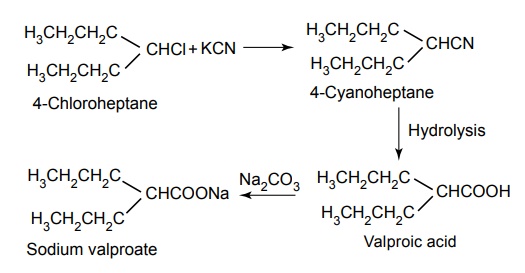
Synthesis
Mode of action: Valproate produces reduction in calcium channel influx and it also prolongs the transient activation time of inactivated sodium channels. Another potential mechanism contributed is to involve in the metabolism of GABA, which is an inhibitory neurotransmitter, by stimulating the GABA synthetic enzyme glutamic acid decarboxylase and inhibits the GABA degradative enzyme GABA transaminase.
Metabolism of valporic acid: It is metabolized by a conjugation of carboxylic acid group and oxidation on one of the hydrocarbon chains.
Properties and uses: It is a white or almost white crystalline powder, hygroscopic, very soluble in water, slightly to freely soluble in alcohol. It has been reported that there is a strong correlation between the anticonvulsant potency of valproate and other branched-chain fatty acids and their ability to reduce the concentration of cerebral aspartate. It has been effective in partial, generalized, and absence seizures.
Assay: Dissolve the sample in anhydrous acetic acid and titrate with 0.1 M perchloric acid. Determine the end point potentiometrically.
Storage: It should be stored in well-closed airtight containers.
Dosage forms: Sodium valproate oral solution I.P., B.P., Sodium valproate tablets I.P., B.P., Gastro-resistant sodium valproate tablets B.P.
Related Topics
