Chirality
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Stereochemical and Conformational Isomerism
Because of the tetrahedral geometry of saturated carbon and the associated three-dimensional properties, molecules can have chirality as one stereochemical feature.
CHIRALITY
Because
of the tetrahedral geometry of saturated carbon and the associated
three-dimensional properties, molecules can have chirality as one
stereochemical feature. Any object is chiral if it is different
(nonsuperimposable) than its mirror image. Likewise a molecule is chiral if it
is nonsuperimposable on its mir-ror image. This requirement does not consider
conformational changes (rotations about single bonds) as valid conditions for
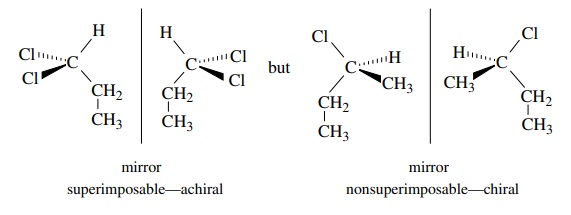
nonsuperimposability. Thus, for the molecules below, the first is achiral (not
chiral) because it is superimposable on its mirror image and the second is
chiral because it is not superimposable on its mirror image.
When
a molecule is chiral, then it will have two isomeric forms called enan-tiomers,
each of which is the nonsuperimposable mirror image of the other. Enantiomers
are distinct stereoisomers because they are compounds that have the same
molecular formula and sequence of bonded elements but which differ in the
spatial arrangement of groups in the molecule. If a molecule is chiral, and
thus has two enantiomers, it usually (but not always) contains at least one
chiral center. In organic compounds a chiral center usually corresponds to an
asymmetric tetrahedral carbon atom.
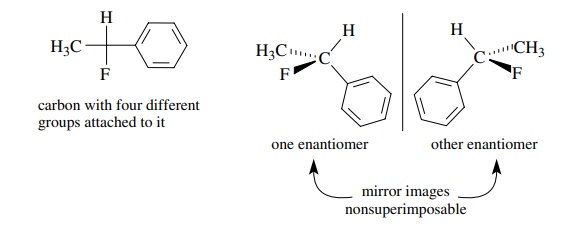
The
most common bonding motif which results in an chiral center is a tetrahe-dral
carbon atom which is bonded to four different groups. A tetrahedral carbon with
four different groups attached is described variously as a chiral center, a
chiral carbon, or an asymmetric center because that carbon lacks symmetry
ele-ments. On the other hand, a tetrahedral carbon with two or more of the same
groups attached automatically has a plane or axis of symmetry associated with
it and so it is achiral (not chiral). When a molecule contains a chiral center,
that molecule lacks symmetry elements and thus is chiral, and it can have two
enantiomers which are nonsuperimposable mirror images of each other.
A
tetrahedral carbon with four different groups attached is also one type of
stereogenic center. A stereogenic center is an atom at which the interchange of
two ligands results in a stereoisomer. It turns out that the interchange of two
groups bonded to a chiral carbon results in a stereoisomer of that molecule. If
there is only a single chiral center in the molecule, then the interchange of
two groups leads to the enantiomer of that molecule.
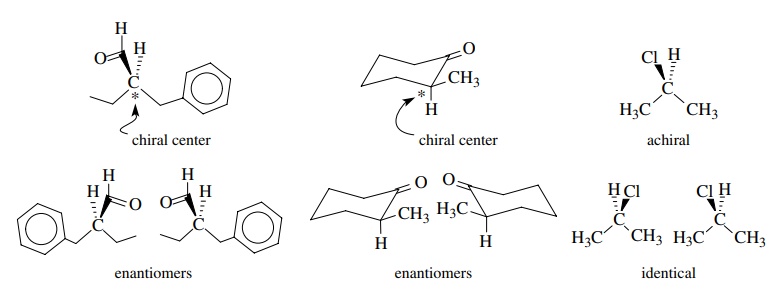
The
four different groups attached to a chiral carbon can be different elements,
isotopes, or functional groups, and chiral centers can be present in both
open-chain molecules or cyclic compounds. The recognition of chirality and
chiral centers in molecules is an important step in determining the numbers of
stereoisomers that are possible for a given compound.
Related Topics
