Multiple Stereocenters
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Stereochemical and Conformational Isomerism
When there is more than one chiral center in a molecule, the number of possible stereoisomers increases.
MULTIPLE STEREOCENTERS
When
there is more than one chiral center in a molecule, the number of possible
stereoisomers increases. Since each chiral center can have either the R or S
configuration, for a molecule of n
chiral centers, there will be 2n
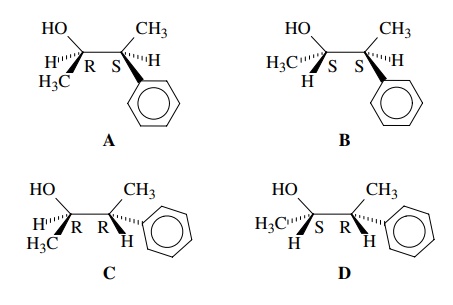
possi-ble stereoisomers. Thus 3-phenyl-2-butanol has two stereogenic centers
and four possible stereoisomers. These are shown below with the configuration
of each chiral center designated.
The
configuration of each chiral center can be determined in the usual way, but
there is a much faster way to draw the four stereoisomers. First draw a
stereostructure of the molecule such as A
and then go through the process of determining the R or S configuration. Each
chiral center is a stereogenic center in that the interchange of two ligands
results in a stereoisomer. Since each chiral center can only be R or S and the
configuration of a particular chiral center can be changed merely by exchanging
any two valences, the remaining stereoisomers of A can now be generated easily by switching valences of one
stereocenter (B), then the other (C), then both (D). Furthermore,
if the relative configurations (R,S) are known for A, then the configurations of the other stereoisomers are
immediately known since exchanging any two valences inverts the configuration
(R goes to S). For 3-phenyl-2-butanol, structure A has the configuration (2R,3S)-3-phenyl-2-butanol. (The
stereochemical information is denoted by the number of the carbon and its
configuration enclosed in parentheses before the name of the compound.) By
exchanging the hydrogen and methyl groups C-2 of A, the configuration of C-2 is inverted and isomer B is produced, the 2S,3S isomer.
Exchange of the hydrogen and phenyl groups at C-3 of A inverts the configuration of C-3 and isomer C is produced, the 2R,3R isomer. Exchanging the hydrogen and methyl
group at C-2 and the hydrogen and phenyl group at C-3 of A gives isomer D, the
2S,3R isomer.
While
the exchange of the methyl and hydrogen groups at C-2 of A was used to invert the configuration, the same configurational
change at C-2 of A could be
accomplished by exchanging any two valences, such as hydrogen and hydroxyl or
hydroxyl and methyl. The same is true for configurational changes at C-3.
Exchange of any two ligands will invert the configuration of a tetrahedral
stereogenic center.
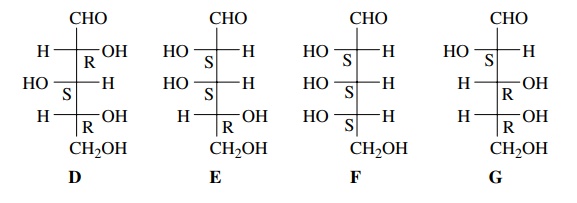
Thus,
given the configurations for the three chiral centers of an aldopentose such as
D, one can rapidly write down the
configurations of the chiral centers of E, F, or G,
which
are stereoisomers of D, without
having to apply the Cahn – Ingold – Prelog rules for each isomer. This is done
by simply noting which substituents are switched relative to those in D. If the configuration is switched
from that in D, then the designation
(R,S) will also be switched from that in D.
Isomers D–G are only four stereoisomers out of eight possible stereoisomers
for an aldopentose which has three stereocenters (23 = 8).
Now
that we can generate the stereoisomers of compounds with more than one chiral
center, it is appropriate to ask what are the relationships between these
isomers. Thus 2-bromo-3-acetoxy butane has two chiral centers and four
stereoisomers, shown below.
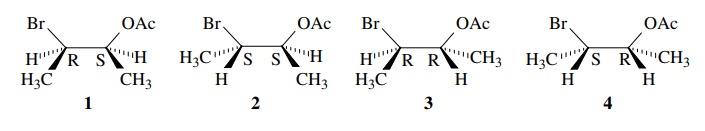
Since
each carbon of 1 is the mirror-image
configuration of the carbons in 4
(i.e., C-2 of 1 is R and C-2 of 4 is S; C-3 of 1 is S and C-3 of 4 is
R), then the molecules themselves are mirror images, but they are
nonsuperimposable. They are thus enantiomers. This relationship can also be
shown by reorienting the molecules to see that they are mirror images,
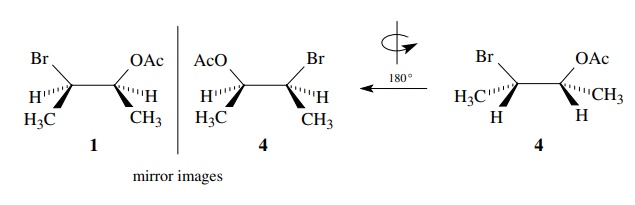
but
nonsuperimposable and therefore are enantiomers.

A
similar analysis reveals that 2 and 3 are also enantiomers. Comparison of
any other pairs of stereoisomers, 1
and 2, for example, shows that they
are not mirror images: The C-2 of 1
is R and C-2 of 2 is S but C-3 of both 1 and 2 is S. Isomers 1 and 2 are also not superimposable. So 1 and 2 are a second
type of stereoisomer and are nonsuperimposable, non-mirror images called
diastereomers. Diastereomers have the same molecular formula and sequence of
bonded elements but different spatial arrangements and are nonsuperimposable,
non-mirror images.
A
third type of stereoisomer occurs when a molecule with several stere-ogenic
centers contains an internal plane of symmetry. This usually happens when two
of the stereogenic centers are attached to the same four different valences.
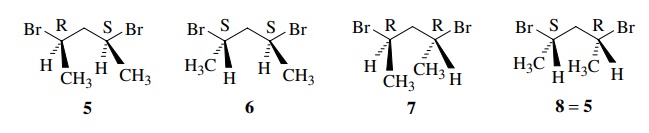
For example, 2,4-dibromopentane has two stereogenic centers and thus four
stereoisomers, 5–8. It is easily
seen that 6 and 7 are enantiomers, 5 and
6 are diastereomers, and so on.
However,
5 and 8 are identical. Although there are two chiral centers in 5 (and 8), the molecule itself is achiral because it contains an internal
mirror plane. Thus it has a plane of
symmetry. Structure 8 is superimposable
on 5 by a 180◦ rotation and thus is
the same compound. This molecule is called a meso isomer, a compound which
contains chiral centers but itself has a plane of symmetry. Even though
2,4-dibromopentane has two stereogenic centers, there are really only three
stereoisomers, a pair of enantiomers and a meso compound which is
diastereomeric with the enantiomeric pair.
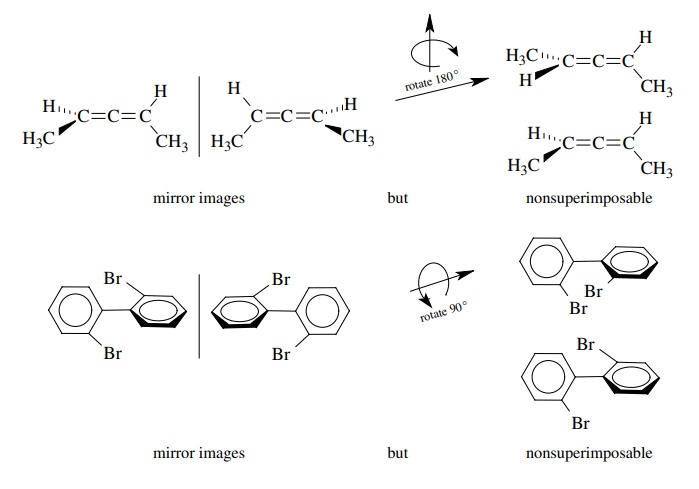
It
is clear from the above examples that the presence of chiral centers in
molecules leads to stereoisomers. There is another type of molecule which
itself is chiral but has no chiral center. The molecular chirality arises from
the presence of a screw axis in the molecule. Allenes and biphenyls are common
examples of such compounds, and because they are chiral, they exist as enantiomers.
We
have seen that when more than one stereocenter is present in a molecule, both
enantiomers and diastereomers are possible. Distinguishing between enan-tiomers
requires the relative configurations of each stereogenic center to be
specified. However, to distinguish diastereomers, only the relative spatial
ori-entation of groups needs to be specified. For example, aldotetroses have
two stereocenters and the four stereoisomers are shown below:

The
enantiomeric relationship between D-threose and L-threose is specified by the
2S,3R and 2R,3S configurations (each stereocenter is the mirror image of the
other). Moreover the enantiomeric relationship between D-erythrose and
L-erythrose is clear from the 2R,3R and 2S,3S configurations. However, threose
and erythrose are diastereomers. The different spatial orientation of the –OH
groups extending from the chain in the Fisher projections makes the
diastereomeric relationship obvious without the need for specifying the
configuration; that is, they are clearly nonsuperimposable and non-mirror
images.
By
extension, other diastereomeric pairs of molecules which contain two adja-cent
stereogenic centers can be designated as threo or erythro depending on whether
substituents extend to opposite (threo) or the same (erythro) sides of the
Fisher projection of the molecule. For example,
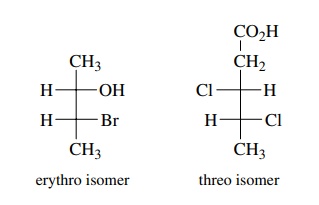
The
threo and erythro designation denotes a diastereomeric relationship of the
isomers. Each threo and erythro isomer will also have enantiomers which will
also have a threo – erythro diastereomeric relationship to each other.
More
recently a new method for designating the stereochemical relationship of
diastereomers has been developed. In this method the carbon backbone is
extended in the plane of the paper, blackboard, or computer screen in the
hor-izontal direction. Groups will extend from this backbone either in front of
the plane or behind it and are designated by bold or dashed bonds,
respectively. If two substituents extend in the same direction, their spatial
relationship is designated syn; if they extend in opposite directions, their
spatial relationship is designated anti.
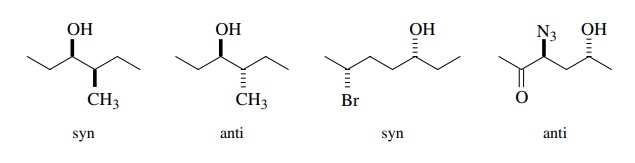
Molecules
which are syn – anti isomers of each other are diastereomers, and there will be
two syn enantiomers and two anti enantiomers. The syn – anti designation is not
restricted to substituents on vicinal carbon atoms as is the threo – erythro
designation and is thus more versatile.
Related Topics
