Formation of Diastereomers
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Stereochemical and Conformational Isomerism
Diastereomers are defined as compounds which have the same molecular formula and sequence of bonded elements but which are nonsuperimposable, non – mirror images.
FORMATION OF DIASTEREOMERS
Diastereomers
are defined as compounds which have the same molecular formula and sequence of
bonded elements but which are nonsuperimposable, non – mirror images. While Z,E
isomers are one subclass of diastereomers which are achiral, the majority of
diastereomeric compounds are chiral compounds which have more than one chiral
center. Furthermore it is important to recall that for a compound with n chiral centers there will be 2n stereoisomers. These will
be divided into 2n /2
pairs of enantiomers, and each pair of enantiomers will be diastereomeric with
the other pairs of enantiomers. This was reviewed earlier in this chapter.
One
of the most direct ways to produce diastereomers is by addition reactions
across carbon – carbon double bonds. If the structure of the olefin substrate
is such that two new chiral centers are produced by the addition of a
particular reagent across the double bond, then diastereomers will result. For
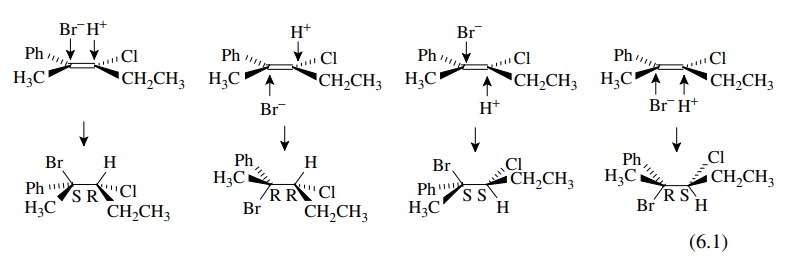
example, the addition of HBr to Z-3-chloro-2-phenyl-2-pentene
produces 2-bromo-3-chloro-2-phenylpentane as a mixture of four diastereomers.
Assuming only Markovnikov addition, the diastereomers are produced by the
addition of a proton to C-3 followed by addition of bromide to the carbocation
intermediate at C-2. Since the olefin precursor is planar, the proton can add
from either face, and since the carbocation intermediate is also planar and
freely rotating, the bromide can add to either face to give diastereomeric
products. The possibilities are delineated schematically (but not mechanistically)
below.
Now
even though there are four possible stereoisomers that can be produced, they
will not necessarily be formed in equal amounts. Diastereomers are not equal in
terms of their energies; consequently, reactions which produce diastere-omers
reflect these energy differences in the various transition states and therefore
proceed at different rates. Thus diastereomers are normally formed in unequal
amounts. This is a very important concept since it provides the kinetic basis for
the stereoselectivity found in many different organic reactions. Restating this
idea, if reactions produce diastereomers and thus proceed via diastereomeric
transition states, then the energy barriers for the formation of individual
diastereomers will be different, the rates of formation of individual
diastereomers will be different, and they will be formed in unequal amounts.
The greater are the differences in the energy barriers, the greater will be the
differences in rates and the more stereoselective will be the reaction. In
contrast to HBr addition, which gives a mixture of diastereomers, there are a
variety of other olefin addition reactions which yield a single diastereomer
from a starting olefin of defined stereochem-istry. Furthermore a starting olefin
with a different stereochemistry will give a different single diastereomer.
Such reactions are described as being stereospecific or highly stereoselective.
The
diastereoselectivity for any process is often reported as a diastereomeric
excess (de%), which is analogous to the optical purity reported for mixtures of
enantiomers. The de% is given by de% = % major diastereomer − % minor
diastereomer. For diastereospecific reactions in which a single diastereomer is
produced, de = 100%, while for reactions in which there is no selectivity and
diastereomers are produced in equal amounts, de = 0%.
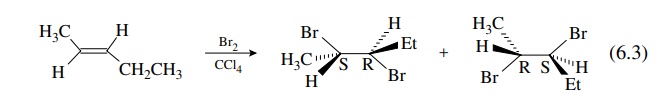
A
typical example of a stereospecific olefin addition reaction is the addition of
bromine to olefins. If cis - 2 - pentene is used as the substrate, only the
2R,3R and 2S,3S pair will be produced (they are enantiomers) .
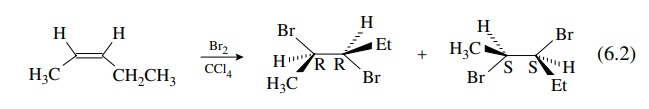
Because
the addition of bromine is stereospecifically trans or anti, one bromine atom
adds to each face of the olefin and can go to either carbon. If t rans - 2 -
pentene is used as the substrate, then only the 2R,3S and 2S,3R pair is
produced (they are also enantiomers.) . However, the pair from cis - 2 -
pentene is diastereomeric with the pair from trans - 2 - pentene.
The
stereospecificity observed in olefin bromination is only possible if the
inherent facial relationship of the olefinic bond is maintained throughout the
addition process and only one bromine atom adds to each face. In bromination,
the electrophilic addition leads to a bridged bromonium ion which not only
maintains the initial olefin geometry but also forces the second bromine to add
from the opposite direction (anti) .
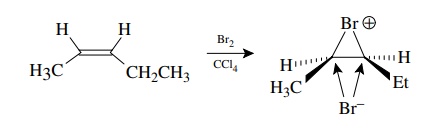
(Contrast
this to the addition of HCl or water to a double bond where the intermediate is
free to rotate so that the olefin geometry is lost and both the proton and the
nucleophile can add to either face).
Other
olefin additions which proceed via bridged intermediates should show similar
stereospecificity and addition should occur anti. Chlorination of olefins is an obvious analogy to bromination, but the
addition of sulfenyl chlorides, oxymercuration, and expoxidation/hydrolysis all
give stereospecific anti addition across the double bond because bridged
intermediates are involved.
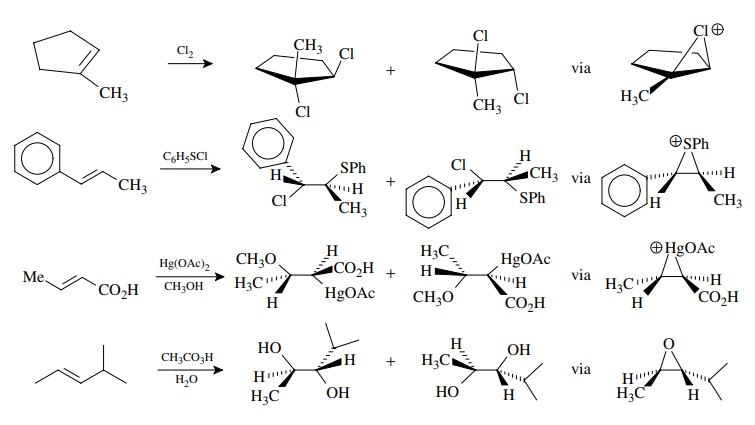
There
are other stereospecific olefin addition processes which occur with cis or syn
stereochemistry. Common examples include catalytic hydrogenation,
hydroboration/oxidation, and dihydroxylation using osmium tetroxide. The
stere-ospecificity of these syn additions requires that the facial properties
of the olefinic bond be maintained throughout the addition process and that both new bonds are formed to
the same face of the olefin. This is normally accomplished by a concerted syn
addition to the π system.
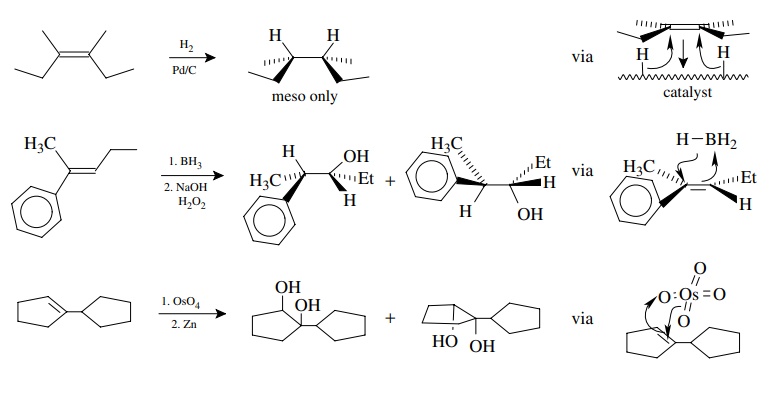
Stereospecificity
in hydrogenation is gained by a surface-mediated delivery of the hydrogen atoms
to one face of the olefin. Stereospecificity in both hydrobora-tion/oxidation
and osmium tetroxide/reduction results from a concerted addition to one face of
the π system. This mode of addition
guarantees that both new bonds are formed on the same face of the olefin.
Although the reagents can add to either face of the olefin, this leads only to
enantiomers of a single diastereomer. The concerted addition is the key feature
which assures syn selectivity.
Another
type of stereoselectivity is possible when a new chiral center is pro-duced in
a molecule which already contains one or more chiral centers. A typical example
of such a process would be addition to an aldehyde or ketone which already
contains a chiral center.
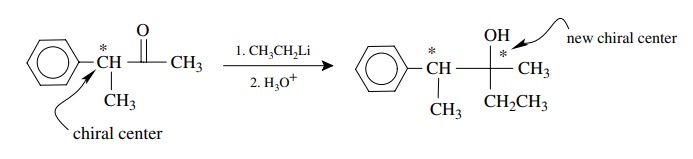
To
understand the stereoselectivity that might be observed, it is first necessary
to delineate the stereochemical possibilities. The existing chiral center can
be either R or S or both. Addition to the carbonyl group can potentially occur
from either face since it is planar. Thus, if the existing chiral center is of
the R config-uration, the products of addition will have either the R,R or R,S
configurations and are diastereomers. If the starting material has only the R
configuration, each diastereomer is optically active because only one
enantiomer is produced. If the existing chiral center is of the S
configuration, the products of addition can be either S,S or S,R diastereomers.
Each diastereomer will be optically active, and they are enantiomers of the
diastereomeric pair formed from the R configura-tion of the precursor. If the
starting material is a racemic mixture, then all four stereoisomers will be
produced —two sets of enantiomeric diastereomers. (That is, the R will give R,R
and R,S and the S will give S,S and S,R.) The product mixture will be optically
inactive.
A given chirality of the starting material gives two diastereomers, and it is normal to find that these two diastereomers are not produced in equal amounts. Because the two diastereomeric products are of different energies, the diastere-omeric transition states leading to them will be of different energies, the rates of their formation will be different, and they will be produced in unequal amounts. If one diastereomer is produced in excess of the other, the reaction is diastereos-elective. If only one diastereomer is produced, the reaction is diastereospecific or highly diastereoselective. The same analysis would apply if the starting material is racemic. The reaction would still produce two diastereomers and each would be formed as a pair of enantiomers, and the same diastereoselectivity would be observed.
As
stated previously, the addition of nucleophiles to chiral carbonyl com-pounds
is a very common type of reaction which produces diastereomeric mix-tures. The
diastereoselectivity varies with the reagents and conditions. Some examples are
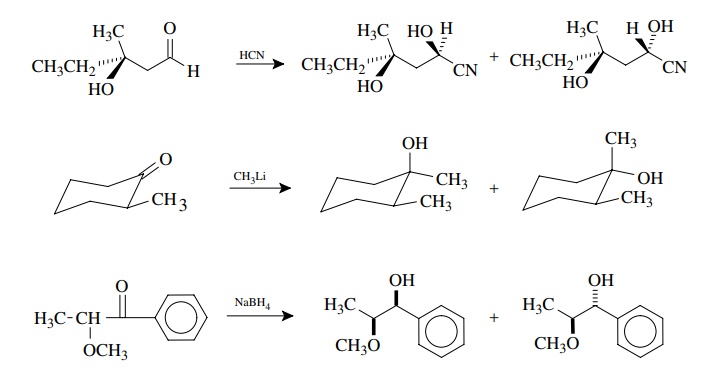
By
analogy, the formation of diastereomers is observed for additions to other
trigonal systems, such as olefins, which have a chiral center elsewhere in the
molecule. In these cases, if optically active starting materials are used, then
the diastereomers will be optically active. If racemic starting materials are
employed, the diastereomeric mixture will be optically inactive. In either case
it is common to find different amounts of the two diastereomers.
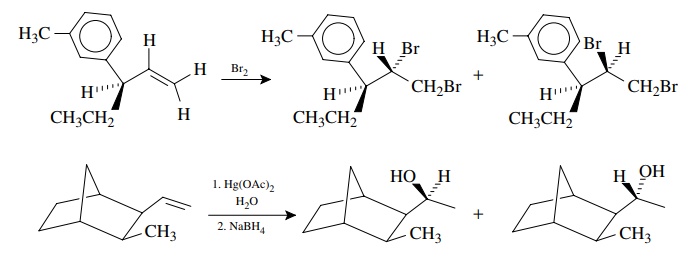
The
formation of diastereomers is also possible when two new chiral cen-ters are
produced from achiral starting materials. A pertinent example is found in
aldol-type reactions between enolates and carbonyl compounds. The achi-ral
enolate and the achiral aldehyde or ketone gives a product with two new chiral
centers. Thus there can be two diastereomers produced, syn and anti, and
because there is no initial chirality, each diastereomer will be produced as a
racemic mixture of enantiomers. The syn and anti diastereomers will usually not
be produced in equal amounts.

Factors
which influence the stereoselectivity of organic reactions have been under
intense investigation recently because of the increasing requirement and
profitability of producing stereoisomerically pure compounds. A great deal of
progress has been made, but even more remains to be accomplished. The specific
contributors to stereoselectivity in individual reactions will be discussed as
they are encountered. At this point it is important to be aware of the
stereochemical variations that are possible.
Related Topics
