Conformational Energies
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Stereochemical and Conformational Isomerism
The energetic consequences of chair – chair interconversions in substituted cyclo-hexanes are related to the interconversion of axial and equatorial valences.
CONFORMATIONAL ENERGIES
The energetic consequences of chair – chair interconversions in substituted cyclo-hexanes are related to the interconversion of axial and equatorial valences. Because axial groups undergo 1,3 diaxial interactions which increase the energy of the molecule, the obvious energy preference is for groups larger than hydrogen to occupy equatorial positions. The relative energies of various chair conformers of multisubstituted cyclohexanes can thus be evaluated by noting the numbers of 1,3 diaxial interactions in each of the conformers.

For
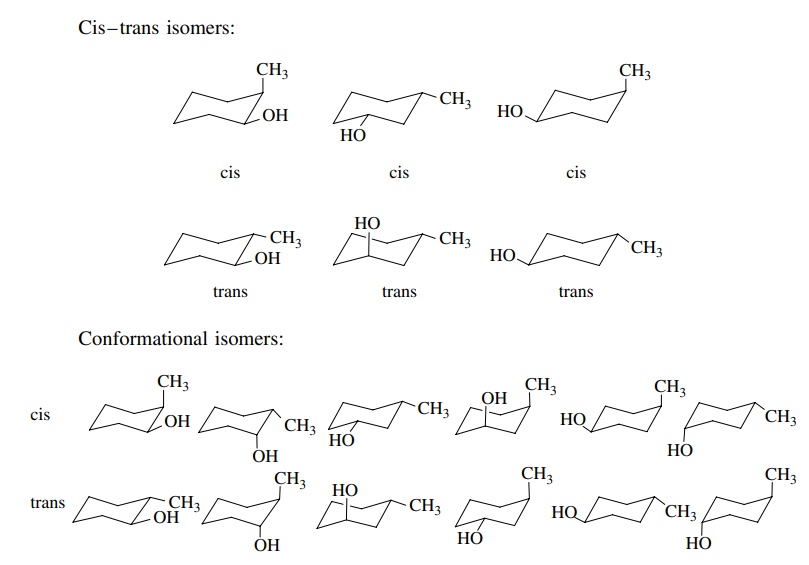
example, trans-1,2-dichlorocyclohexane
has diaxial and diequatorial chair forms. The diaxial conformer should be less
stable because it has two sets of 1,3 diaxial interactions between the
chlorines and the axial protons. Knowing the equatorial– axial preference for a
single chlorine substituent on a cyclohexane is 0.52 kcal/mol (2.2 kJ/mol), one
can predict that the diequatorial isomer is favored by 1.04 kcal/mol (4.4
kJ/mol). cis-1,3-Dichlorocyclohexane
has two chair forms with two equatorial chlorines and two axial chlorines,
respectively. The diaxial isomer should be more than 1 kcal/mol higher in
energy than the diequatorial isomer because the 1,3 diaxial interactions
between two axial chlorines should be more severe than the 1,3 diaxial
interactions between an axial chlorine and axial hydrogens. In contrast, trans-1,3-dichlorocyclohexane has a
single axial chlorine in either chair conformer; thus the two chair forms are
of the same energy.
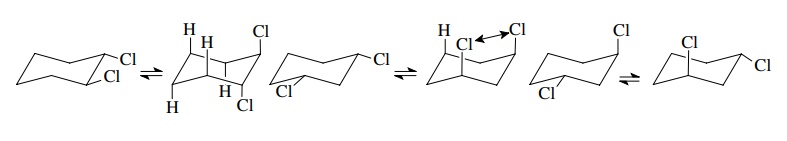
Similarly
trans-4-methylcyclohexanol has
diequatorial and diaxial substituted chair forms. It is thus predicted that the
former should be about 2.7 kcal/mol (11 kJ/mol) more stable than the latter
because an axial methyl is less stable by 1.8 kcal/mol (7.3 kJ/mol) and an
axial OH group is less stable by 0.9 kcal/mol (3.8 kJ/mol) than their
equatorial counterparts.
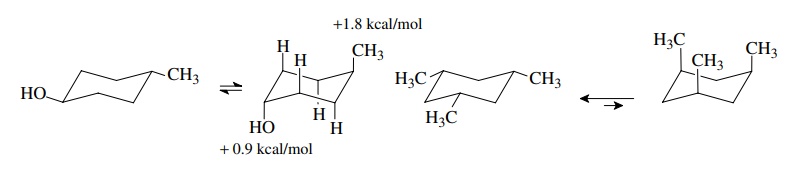
Similar
qualitative assessments can be made for more highly substituted cyclo-hexanes.
It is found that cis,cis-1,3,5-trimethyl
cyclohexane exists in only one chair form. This must be the triequatorial
isomer because the other chair form has three axial methyl groups interacting
on the same side of the ring. This should cause severe steric interactions and
be much less stable than the all-equatorial isomer. In fact, the energy
difference between the all-equatorial and the all-axial isomer should be
greater than 5.4 kcal/mol (3 ×
1.8 kcal/mol). This can be esti-mated
by noting that an axial methyl group is less stable by 1.8 kcal/mol when the
other axial valences with which it interacts are protons. The 1,3 diaxial
inter-actions should be even greater if the other axial valences hold groups
larger than protons; thus the energy difference should be greater than for
three axial methyl groups or greater than 3 × 1.8 = 5.4 kcal/mol.
Since
1,3 diaxial interactions are the major factor which increases the energy of
conformations with axial substituents, it is reasonable to expect that larger
groups would have more severe steric interactions, causing the energy
difference between the axial and equatorial position to increase. That is, the
larger the group, the larger is the 1,3 diaxial interactions with axial protons
and the greater is the energy difference between the axial and equatorial
forms.
This effect is clearly seen by comparing methylcyclohexane and t-butylcyclohexane. The axial– equatorial energy difference is 1.8 kcal/mol for the methyl group while it is 4.9 kcal/mol for the t -butyl group. This is because the t -butyl group is much larger than a methyl group, and 1,3 diaxial interactions are much stronger. In fact, these interactions are so large that the t -butyl group has been employed to anchor the particular chair conformation that has the t -butyl group equatorial. The other chair form would be much higher in energy and virtually unpopulated; thus the chemistry that is observed arises from reactions of a single conformation of the t -butyl-substituted ring.
Related Topics
