Esters
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Group Synthesis
Derivatives of carboxylic acids are generally made from the carboxylic acid.
ESTERS
Derivatives
of carboxylic acids are generally made from the carboxylic acid. The
traditional routes to esters are as follows:
1.
Acid-catalyzed reaction between an acid and an alcohol (Fischer
esterification):
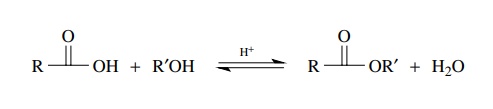
2.
Reaction of an acid chloride (or acid anhydride) with an alcohol:
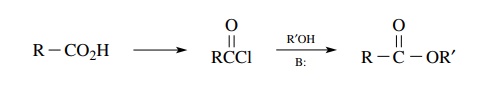
While
still useful for large-scale esterification of fairly robust carboxylic acids,
Fischer esterification is generally not useful in small-scale reactions because
the esterification depends on an acid-catalyzed equilibrium to produce the
ester. The equilibrium is usually shifted to the side of the products by adding
an excess of one of the reactants — usually the alcohol— and refluxing until
equilibrium is established, typically several hours. The reaction is then
quenched with base to freeze the equilibrium and the ester product is separated
from the excess alcohol and any unreacted acid. This separation is easily
accomplished on a large scale where distillation is often used to separate the
product from the by-products. For small-scale reactions where distillation is
not a viable option, the separation is often difficult or tedious. Consequently
Fischer esterification is not widely used for ester formation in small-scale
laboratory situations. In contrast, intramolecular Fischer esterification is
very effective on a small scale for the closure of hydroxy acids to lactones. Here
the equilibrium is driven by the removal of water and no other reagents are
needed. Moreover the closure is favored entropically and proceeds easily.
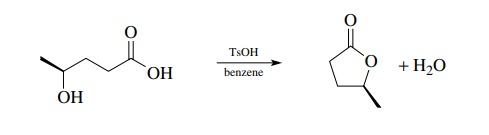
A
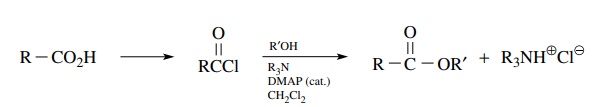
second very common way to convert carboxylic acids to esters is by the reaction
of the corresponding acid chloride with an alcohol. A tertiary amine such as
pyridine or triethylamine is used to scavenge the HCl by-product. It has also
been found effective to add small amounts of N ,N
-dimethyl-4-aminopyridine (DMAP) to the reaction mixture in order to promote
efficient product formation. If the acid chloride is readily available, this is
a very satisfactory preparation. If the acid chloride is not available, a
disadvantage to this method is that a car-boxylic acid must first be converted
to the acid chloride, which must be isolated and purified prior to the
formation of the ester. If the chlorinating agent is not separated from the
acid chloride, the alcohol will also react with the chlorinating agent leading
to a mixture of products that may be difficult to separate.
For
small-scale esterification reactions (<500
mg), the best methods should occur rapidly under mild conditions and only
produce by-products which are easily separated from the reaction products.
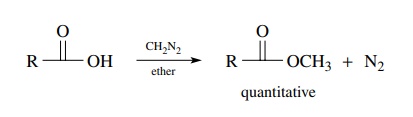
Under these criteria an extremely efficient way to convert acids to methyl
esters is to titrate the carboxylic acid with an ethereal solution of
diazomethane. The methyl ester is produced rapidly and quantitatively, and the
by-product of the esterification is nitrogen. Although diazomethane is a
reactive and explosive compound, solutions of diazomethane can be prepared from
readily available reagents and used safely in the laboratory.
A
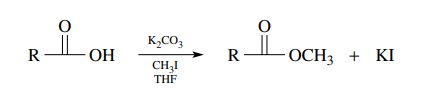
second method to efficiently produce methyl esters of carboxylic acids is to
treat the acid with potassium carbonate and methyl iodide. The methyl ester is
produced under mild conditions and is easily separated from the reaction
by-products. This method is somewhat different in that the ester is formed by a
nucleophilic displacement of iodide by the carboxylate ion. Normally
carboxy-lates are not thought of as good nucleophiles — and they are not— but
methyl iodide is a quite reactive electrophile which matches the poor
nucleophilicity of the carboxylate satisfactorily.
Besides the above methods, many other satisfactory ways to convert acids to esters are commonly encountered.
