Amines
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Group Synthesis
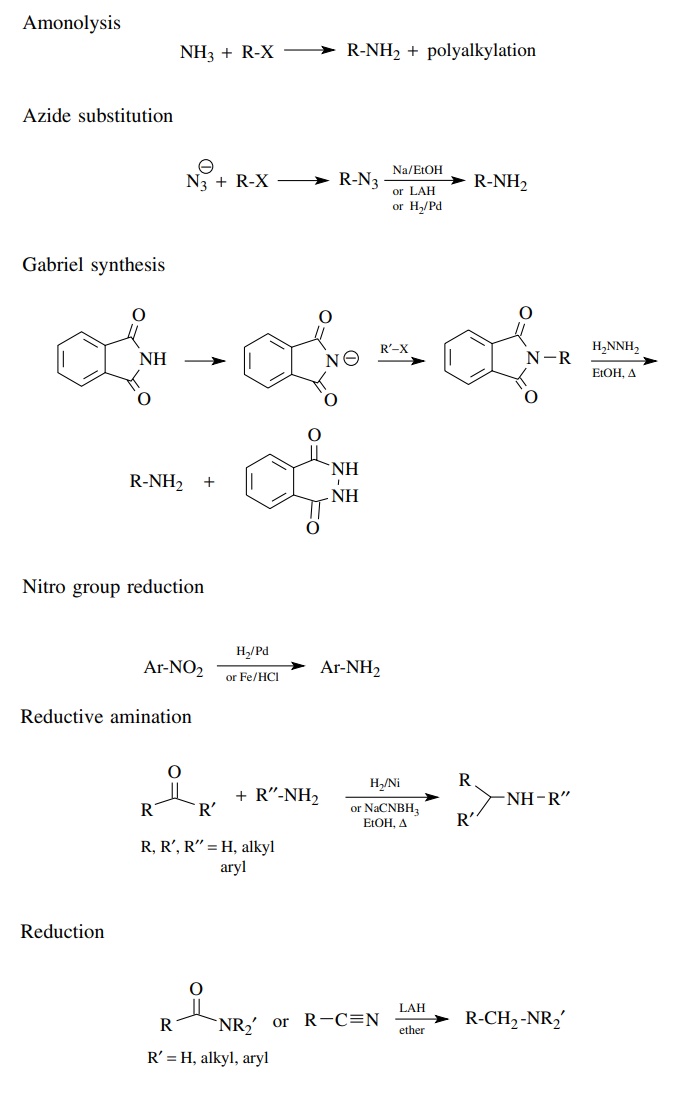
Amines are saturated, nitrogen-containing functional groups that are widely en-countered.
AMINES
Amines
are saturated, nitrogen-containing functional groups that are widely
en-countered. Because the nitrogen atom of amines is basic, nucleophilic, and
oxidizable, some constraints on the preparation of amines result. A collection
of textbook amine preparations includes the following:
Amines
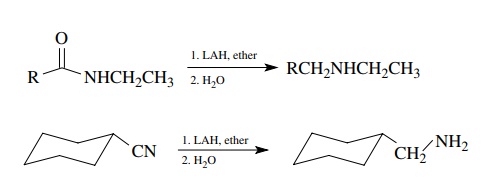
are at the same low oxidation level as alcohols and consequently are easily
prepared by reduction. Amides and nitriles are reduced efficiently by LAH to
amines. Nitriles give only primary amines while amides give 1◦ , 2◦ , or 3◦ amines depending on
the number of carbon substituents on the amide nitrogen. The advantage of this
method is that amides are easy to prepare from acid chlorides and amines while
nitriles are available by displacement reactions.
One
problem with this method is that the workup must be done carefully as the amine
products tend to complex tenaciously with the aluminum salts formed from the
LAH upon workup and thus are not recovered easily. There are standard workups
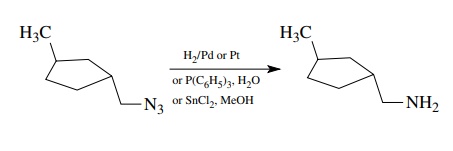
which avoid these issues, but these should be followed carefully. Reduction of
azides by catalytic reduction, phosphine or phosphite reagents, or Sn(II)
chloride are all effective methods. The azides are also available from
displacement reactions and give primary amines upon reduction.
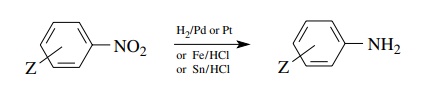
Aromatic
amines are readily prepared by the reduction of aromatic nitro groups by Fe/HCl
or Sn/HCl or by catalytic hydrogenation.
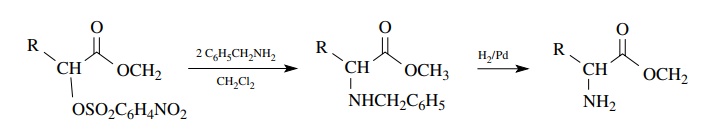
Displacement
reactions are rarely used for the preparation of amines as poly-alkylation
reduces yields and makes product mixtures more complex. However, reaction of
primary amines with primary and secondary sulfonates can provide good yields of
monoalkylated product if care is taken to control the conditions and mode of
addition. Benzylamine is particularly common as a primary amine nucleophile
since the benzyl group can be removed by hydrogenolysis to give a primary
amine.