Nucleophilic Carbon
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
Carbon-centered nucleophiles are those compounds or intermediates which contain an electron-rich carbon atom and thus are capable of donating an electron pair from that carbon atom to an electrophile.
NUCLEOPHILIC CARBON
Carbon-centered
nucleophiles are those compounds or intermediates which contain an
electron-rich carbon atom and thus are capable of donating an electron pair
from that carbon atom to an electrophile. The electron pair that is donated is
found in a filled orbital in the nucleophilic carbon and the electrons are not
tightly bound. Donation to the electrophilic carbon occurs by overlap of the
filled orbital of the donor with an unfilled orbital of the acceptor. The most
common carbon nucleophiles fall into three main classes:
(a)
Organometallic molecules contain a carbon–metal bond which is polarized toward
carbon. As a result the carbon is very electron rich and reacts vigorously with
electrophiles.
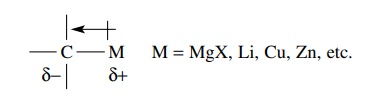
Examples
of organometallic compounds most commonly used as carbon nucle-ophiles include
Grignard reagents (RMgX), organolithiums (RLi), organocuprates (R2CuLi),
and occasionally organocadmiums (R2Cd) and organozincs (RZnBr).
(b)
Enolates are anionic derivatives of carbonyl compounds formed by proton removal
from a position adjacent to the carbonyl group. Resonance delocalization of the
negative charge with the carbonyl group stabilizes enolate anions and makes
them somewhat less reactive than organometallic compounds.
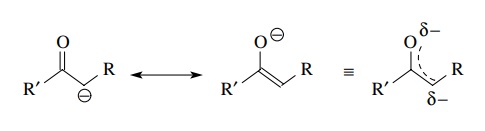
They
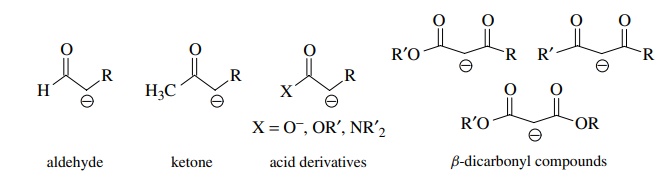
are, however, reactive carbon nucleophiles. Examples of enolates include
anionic derivatives of aldehydes, ketones, acid derivatives, and dicarbonyl compounds.
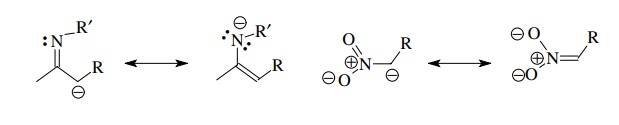
Structurally
related to enolates are anionic derivatives of imines and nitro compounds. The
former are less stable (more reactive) than enolates because nitrogen cannot
support a negative charge as well as oxygen and thus resonance stabilization is
diminished compared to enolate anions. Nitronate anions are much more stable
(less reactive) than enolates because resonance with the nitro group transfers
the negative charge to oxygen where it is stabilized by the formal positive
charge on nitrogen.
(c) A third type of molecule capable of functioning as a nucleophilic carbon equivalent is one that contains an electron-rich π bond. Such species are usually uncharged and function as carbon nucleophiles because the π electrons are less tightly bound and can consequently be donated to good electron acceptors. Enol derivatives are neutral derivatives of carbonyl compounds which have an oxy-gen or nitrogen substituent attached to a carbon–carbon double bond. They are covalent analogs of enolate ions, and because they neutral, they are much less reactive electron donors than enolate anions. In fact, most enol derivatives are stable compounds which can be isolated. In spite of being neutral, however, reso-nance interaction of the lone pairs of the heteroatom with the π system increases the electron density at the β position of the double bond and the double bond is electron rich. Besides the parent enol, examples of enol derivatives of carbonyl compounds include those shown below.
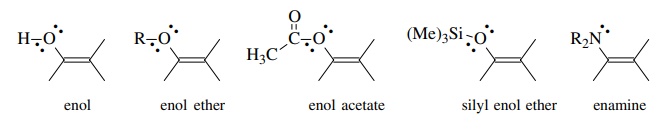
Besides
a heteroatom substituent, which renders a double bond electron rich by
resonance interaction of the lone pairs with the π system, other substituents can also result in π bonds being electron rich and thus
reacting as electron donors. Attachment of substituents less electronegative
than carbon to the double bond increases its π -electron density significantly by an inductive effect.
Vinylsilanes and vinyl stannanes can both be considered to have electron-rich π bonds and have been used as π -electron donors. Allylsilanes, by
virtue of hyperconjugation between the allylic carbon–silicon bond and the π system, also function as good π-electron
donors in many reactions.
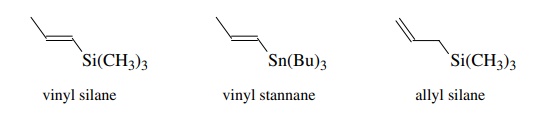
Related Topics
