Reactivity Matching
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
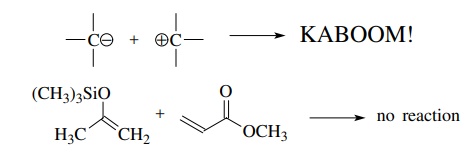
Having defined the types of commonly used carbon nucleophiles and carbon electrophiles, it would seem that if you react any of the carbon nucleophiles (electron donors) with any of the carbon electrophiles (electron acceptors), then a carbon–carbon bond should be formed.
REACTIVITY MATCHING
Having
defined the types of commonly used carbon nucleophiles and carbon
electrophiles, it would seem that if you react any of the carbon nucleophiles
(electron donors) with any of the carbon electrophiles (electron acceptors),
then a carbon–carbon bond should be formed. While this is theoretically true,
it is unworkable from a practical point of view. If, for example, a carbanion
nucle-ophile was reacted with a cationic electrophile, it is unlikely that the
desired carbon–carbon bond formation would be detected, even after the smoke
cleared. Or if a silyl enol ether nucleophile was reacted with an α, β-unsaturated
ester, no reaction could be observed to take place in any reasonable time
frame.
For
carbon–carbon formation to be successful, the reactivities of the nucle-ophile
and electrophile must be matched so that reaction occurs at a reasonable and
controllable rate. Thus we must be able to easily generate both carbon
nucle-ophiles and carbon electrophiles to be able to choose the appropriate
partners for successful C–C bond formation.
Related Topics
