Organometallic Compounds
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
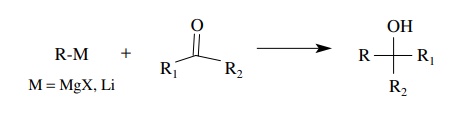
Organometallic compounds such as Grignard reagents and organolithium reagents are very powerful nucleophiles which react with a wide variety of carbonyl compounds.
ORGANOMETALLIC COMPOUNDS
Organometallic
compounds such as Grignard reagents and organolithium reagents are very
powerful nucleophiles which react with a wide variety of carbonyl compounds. In
general, organolithium reagents are more reactive than Grig-nard reagents. Both
types of reagents react rapidly with aldehydes and ketones and yield secondary
and tertiary alcohols after aqueous workup. The new car-bon–carbon bond joins
the organometallic fragment with the carbonyl carbon.
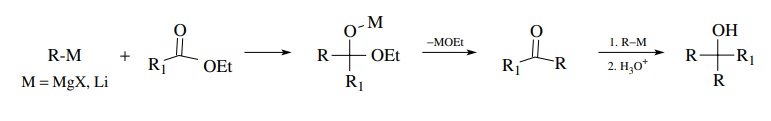
The
tetrahedral intermediate produced by addition of organolithiums or Grig-nard
reagents to esters collapses to a ketone which is more reactive than the
original ester electrophile. A second equivalent adds to give a tertiary
alcohol as the product. This process cannot be controlled by temperature or by
mode of addition since the intermediate product is more reactive than the
starting material.
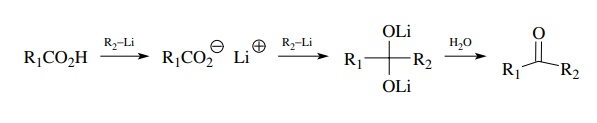
Collapse
of the tetrahedral intermediate can be prevented, however, by reaction of a
carboxylic acid with two equivalents of an organolithium (or a carboxylate salt
with one equivalent of the organolithium). Addition to the carboxylate gives a
dianionic tetrahedral intermediate which has the ketone oxidation state but is
stable to further addition under the reaction conditions. The ketone is
revealed only upon hydrolysis. This is a widely used method for the preparation
of ketones by the formation of a carbon–carbon bond, but it is restricted to
organolithium reagents as the carbon nucleophile. Grignard reagents do not
react with car-boxylates, illustrating they have a slightly reduced
nucleophilicity relative to organolithiums.
Related Topics
