Solved Problems on Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
Questions and answers, Solved Problems on Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles - Organic Chemistry
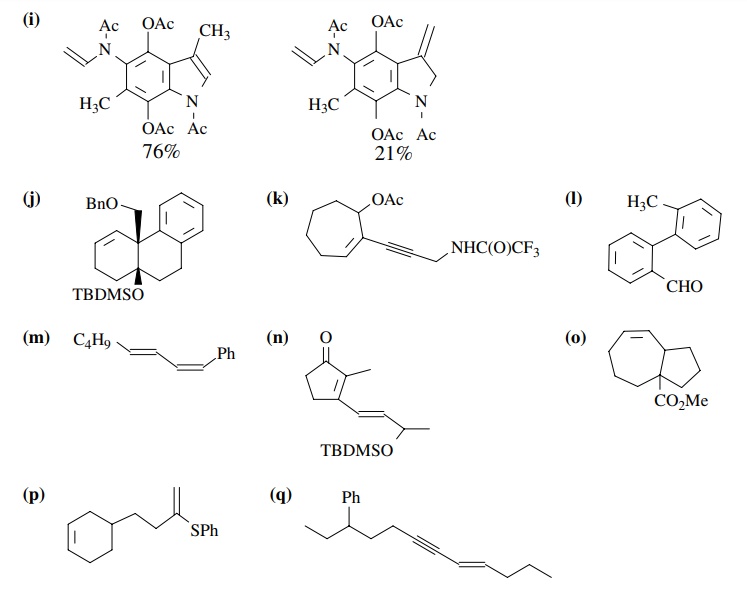
PROBLEMS
8.1. Give the
products of the following reactions. Indicate the new carbon–car-bon bond that
has been formed and identify the carbon nu-cleophile and electrophile.
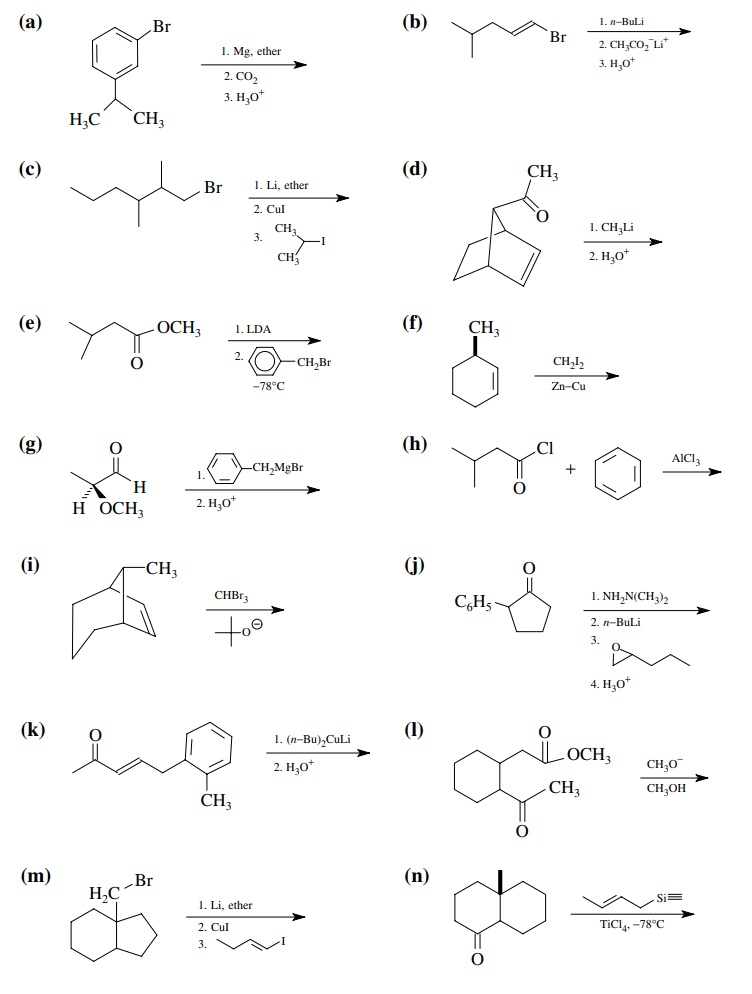
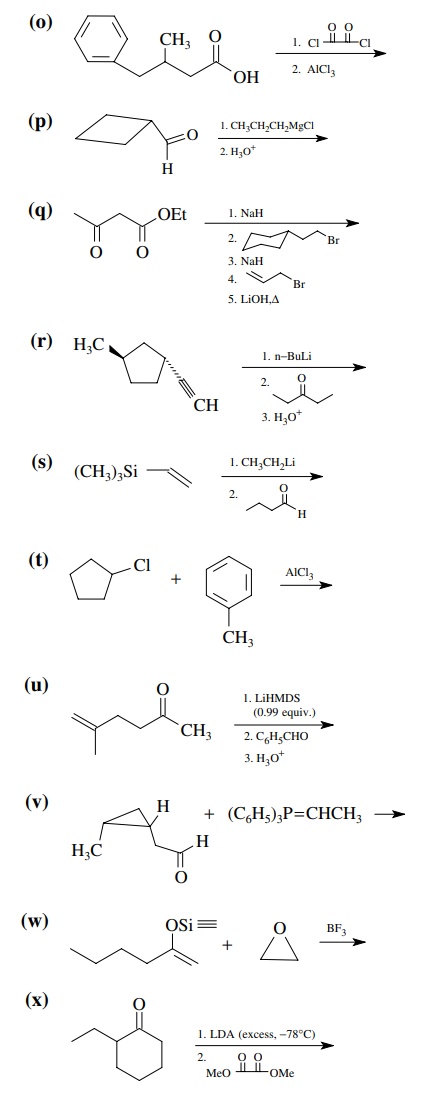
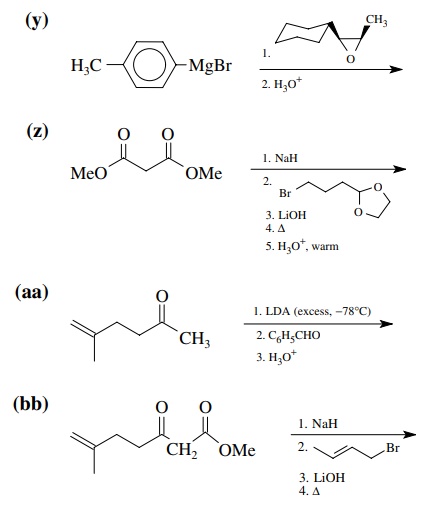
Answer:
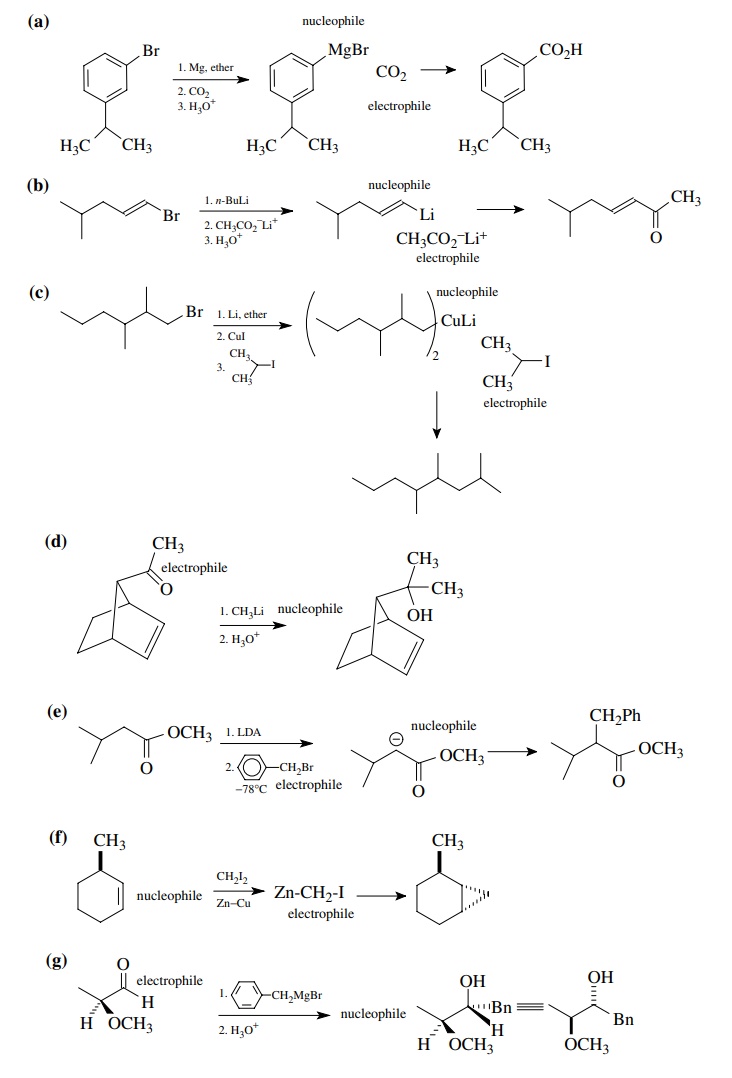
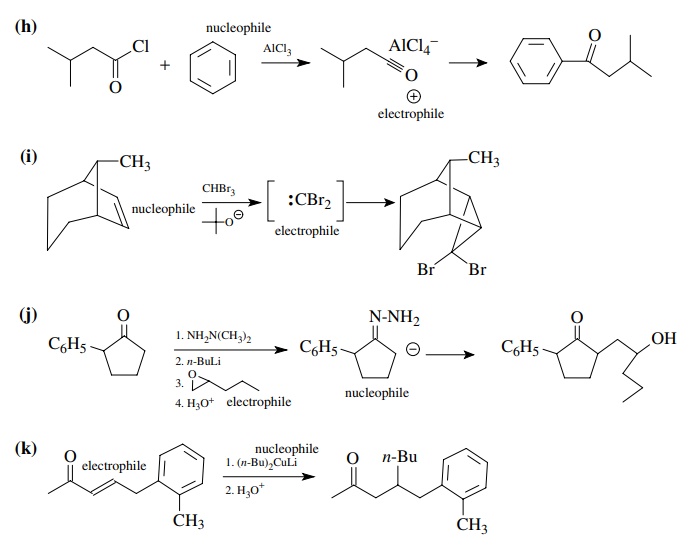
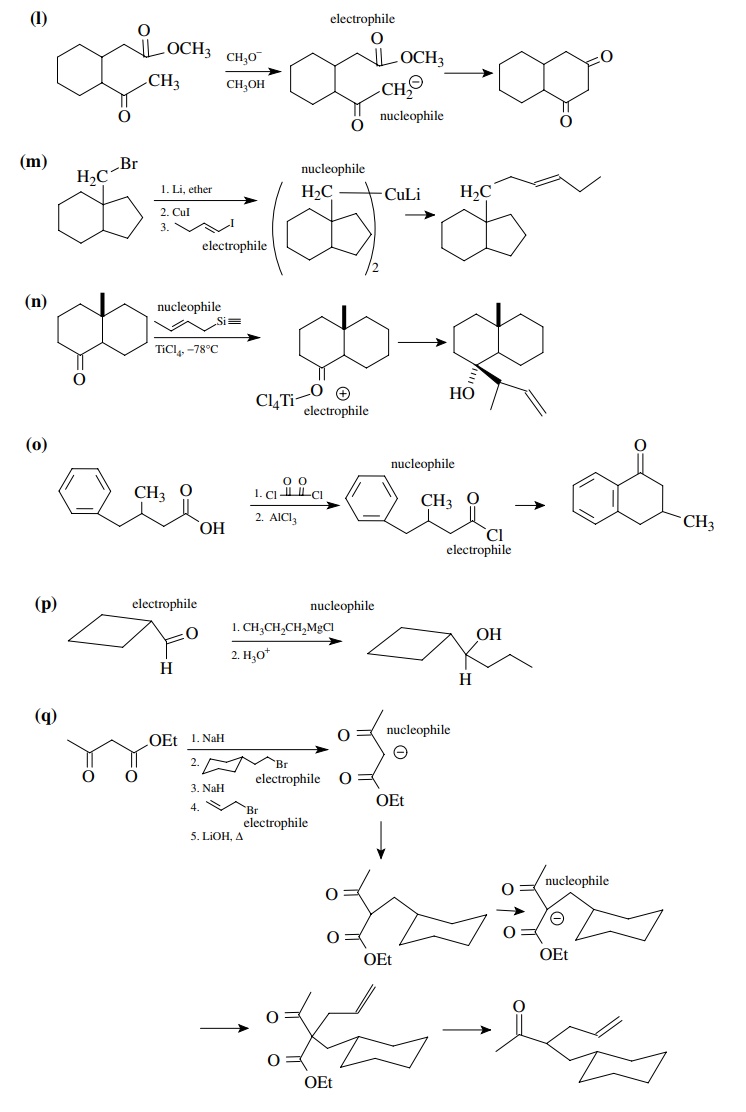
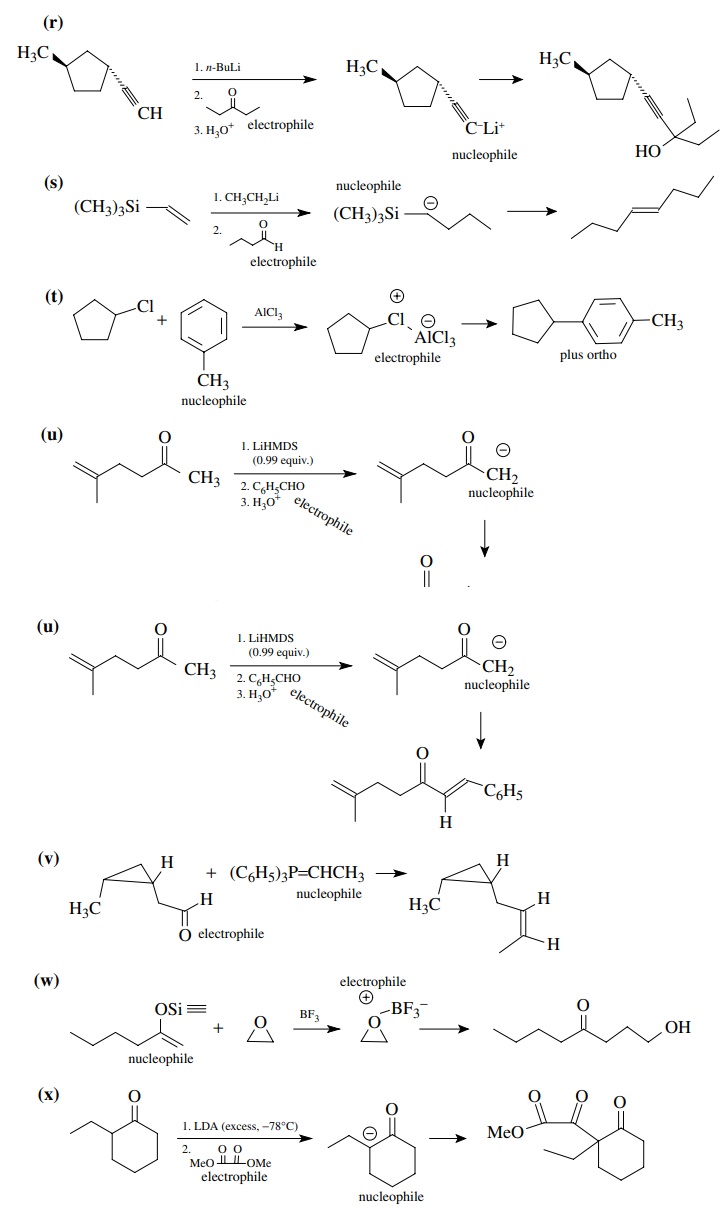
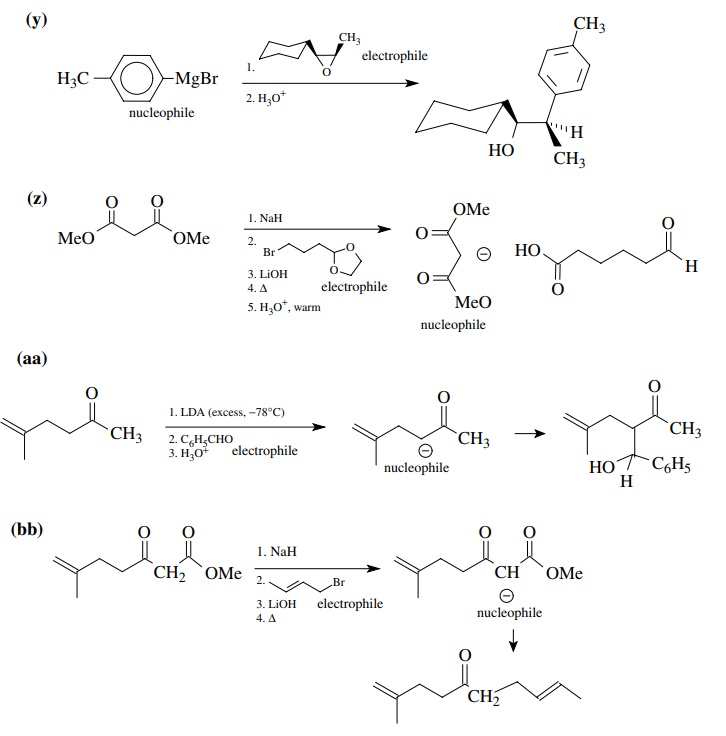
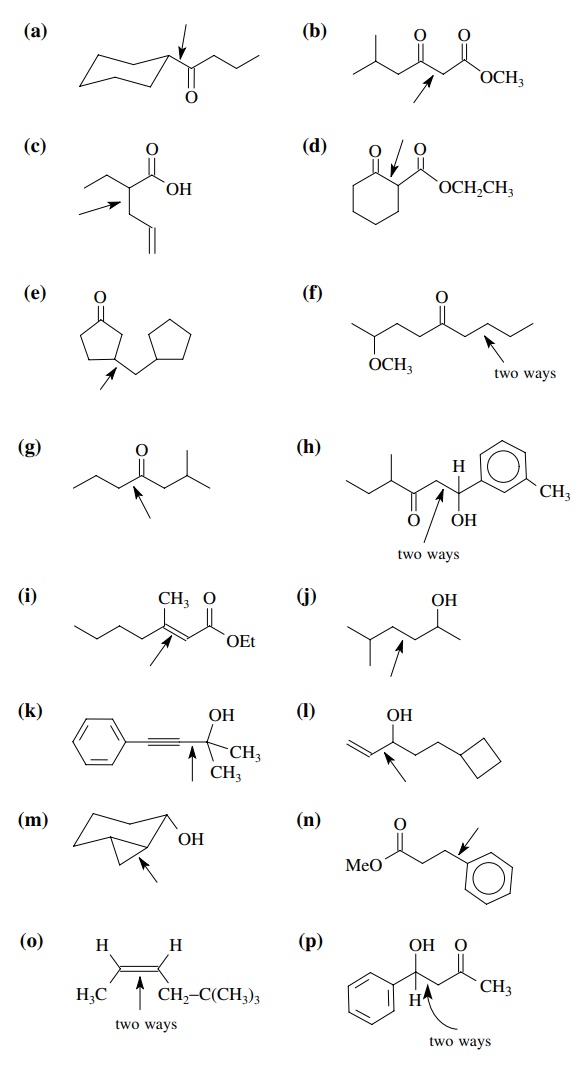
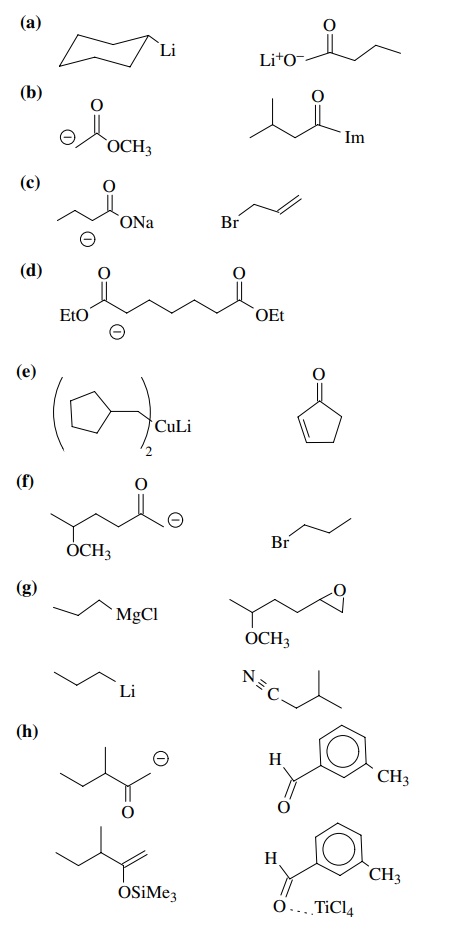
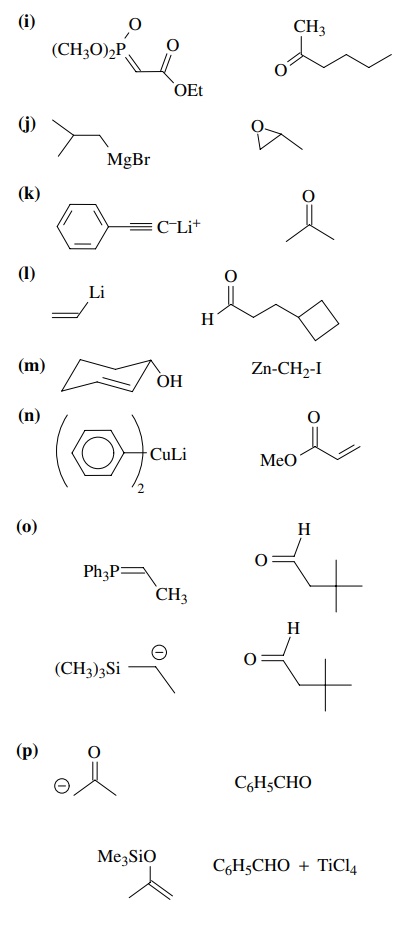
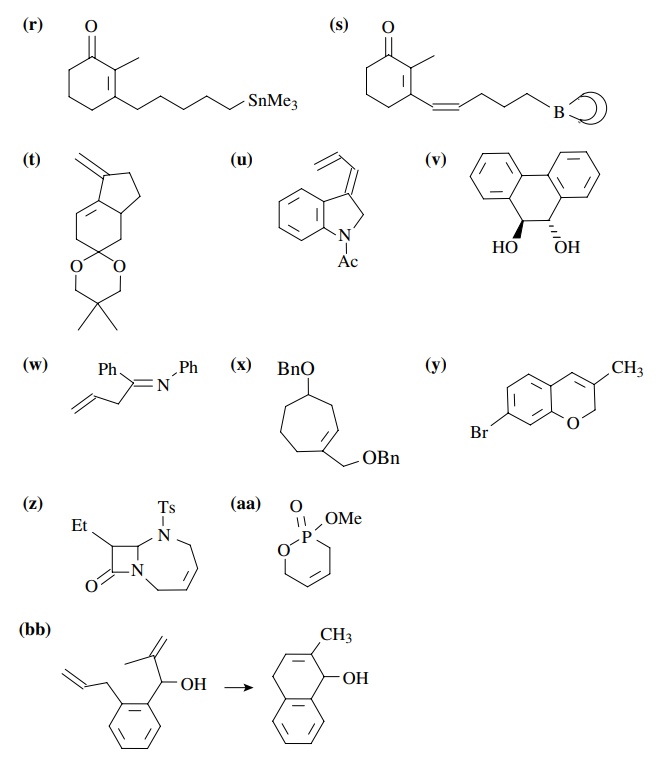
8.2. Give reactions
which would produce the indicated bond in the following compounds. Give the
reacting partners and tell which is the electrophile and nucleophile. Also tell
how you would generate any reactants which are not stable compounds.
Answer:
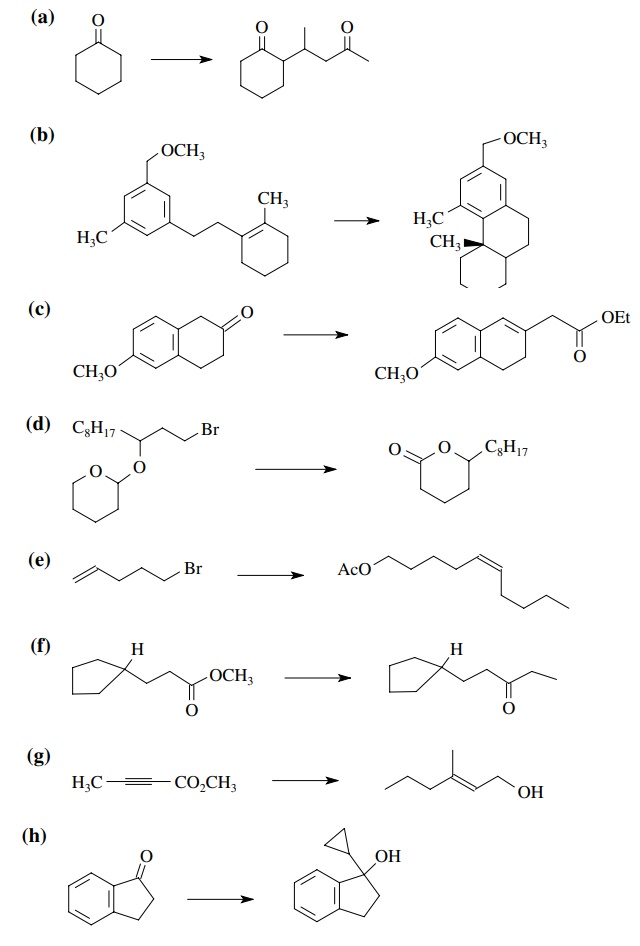
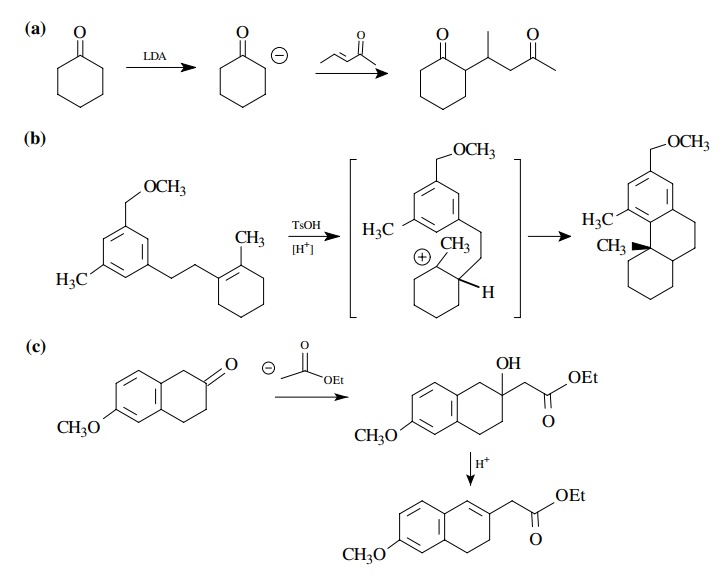
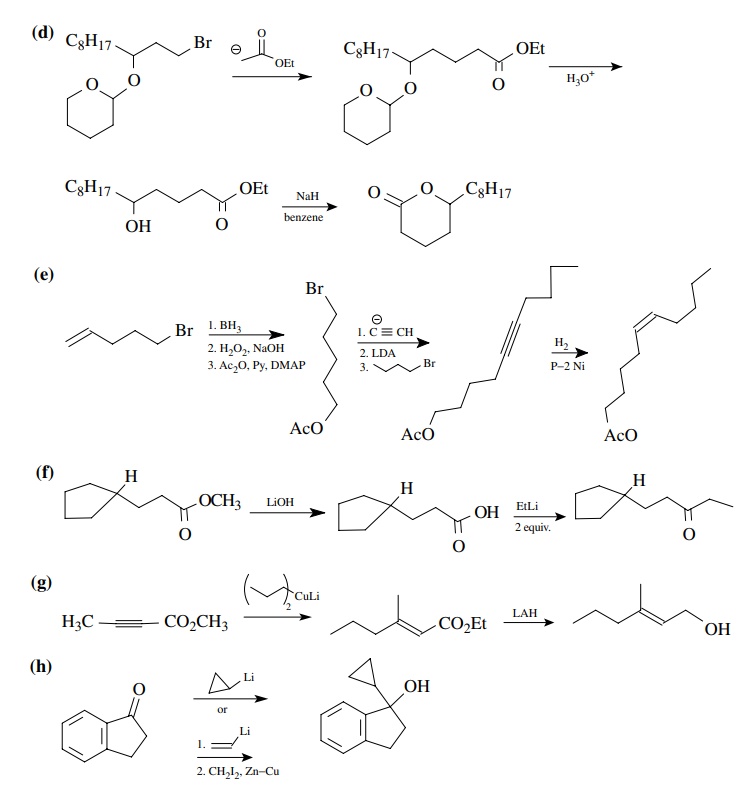
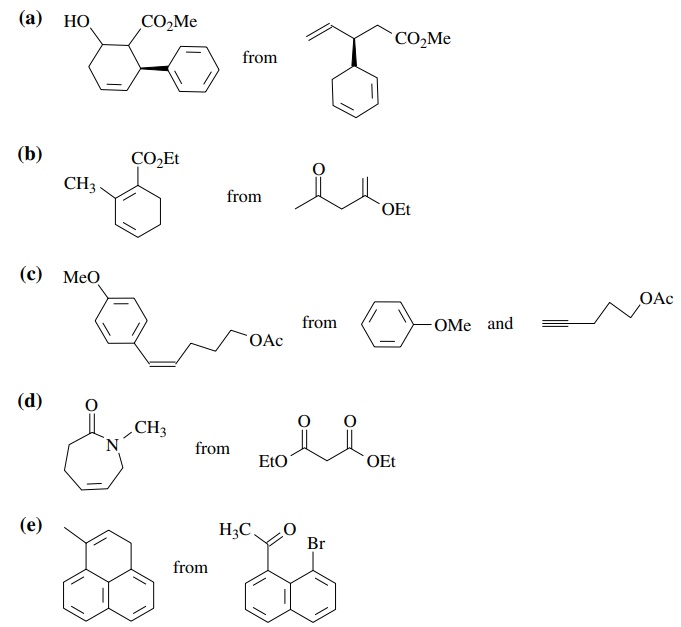
8.3. Show how you
would prepare each of the following molecules from the indicated starting
materials. Where more than one step is required, show each step clearly.
Answer:
8.4. Give the
products of the following reactions. [The reagent Pd(0) is a generic term
referring any one of a number of possible zerovalent palla-dium reagents. The
reagent referred to a Grubbs catalyst is the ruthenium carbenoid species
referred to in the chapter.]
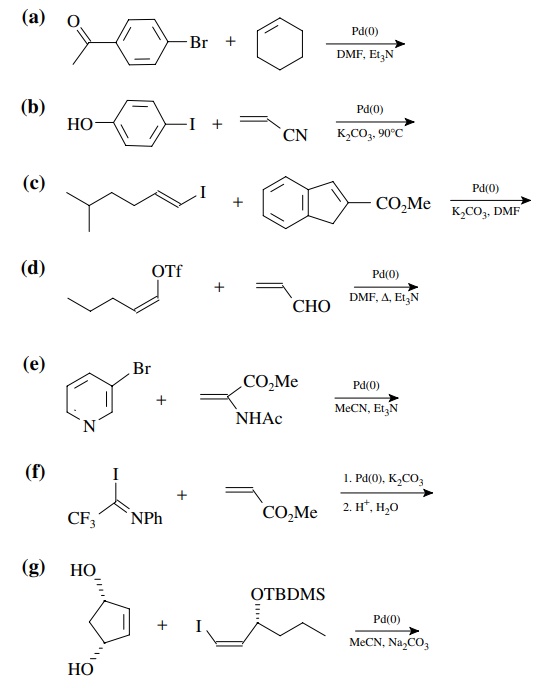
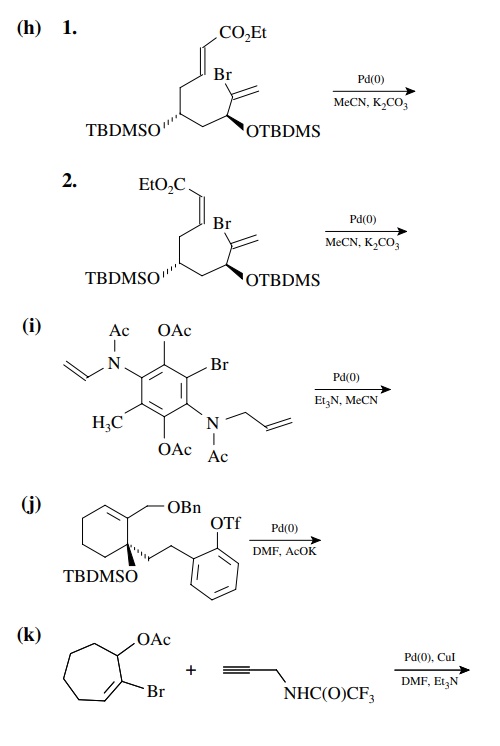
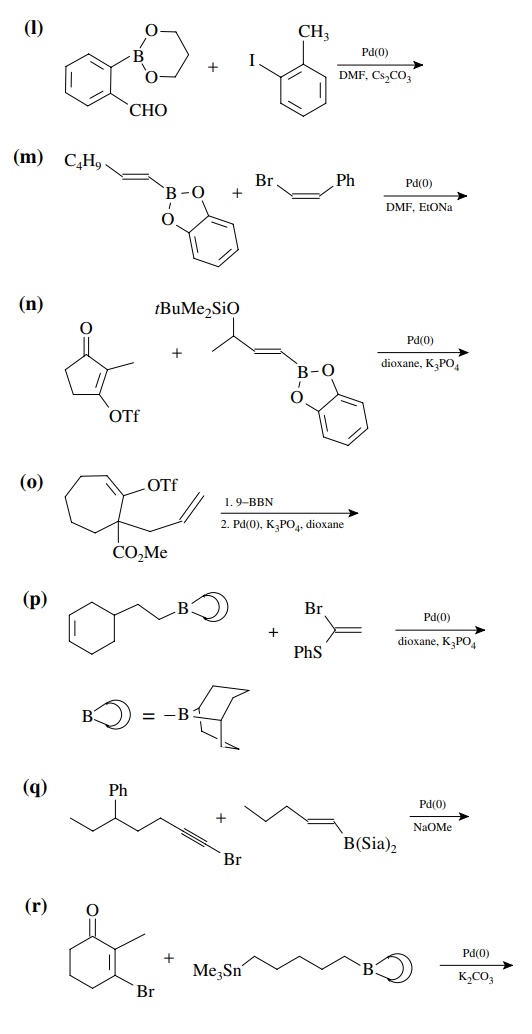
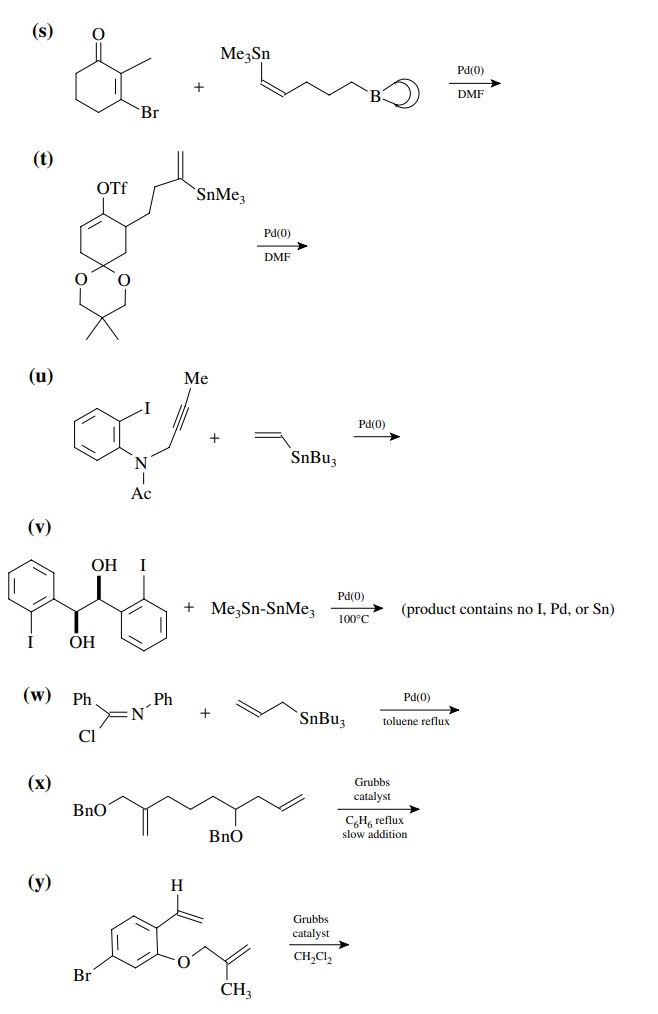
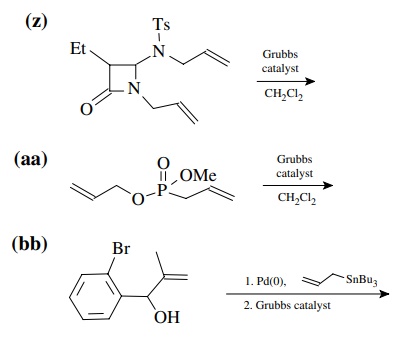
Answer:
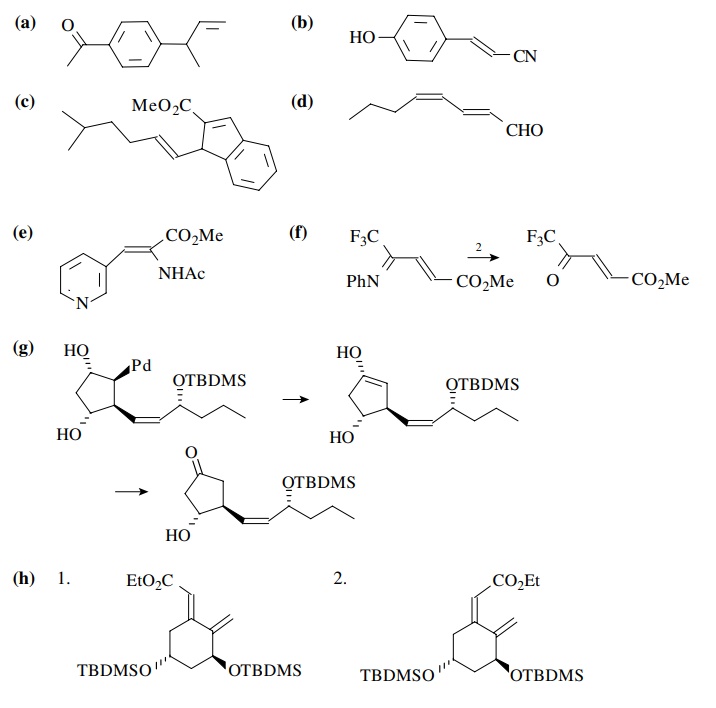
8.5. Show a method
for constructing the following compounds from the indi-cated starting
materials.
Answer:
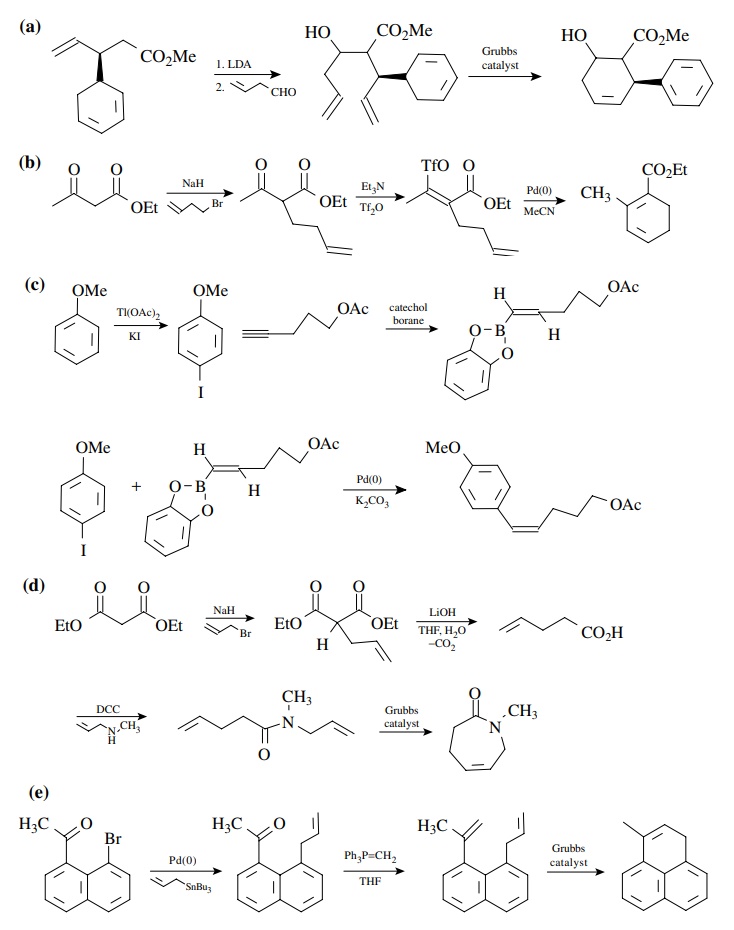
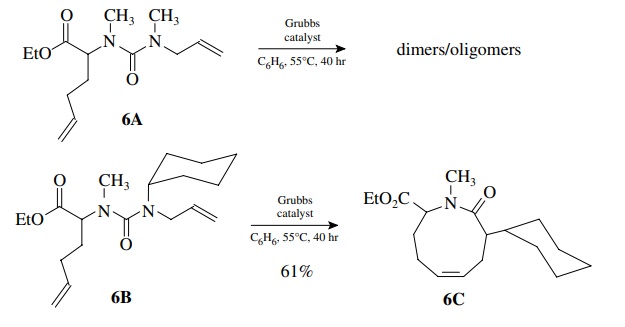
8.6. It was observed
that when urea 6A was treated with Grubbs catalyst under high dilution, only
dimers and oligomers were produced. However, when urea 6B was reacted under the
same conditions, cyclic urea 6C was pro-duced in 61% yield. Provide a
rationalization for these results.
Answer:
The
formation of medium rings requires that the two reacting double bonds be in
proximity to one another and that dimerization is controlled by high dilution.
Since 6A does not cyclize under high
dilution, the rate of cyclization is slow com-pared to dimerization. Placement
of a much larger cyclohexyl group on nitrogen causes cyclization to proceed
much faster, assuming that the dimerization rate is similar for both, which is
a fair assumption. The cyclohexyl group must cause a conformational change
about the urea linkage that causes the two double bonds to be in close
proximity, thus increasing the cyclization rate greatly.
Related Topics
