Cyclopropanation Reactions
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
Formation of two single bonds from a carbon atom is also well known for building up carbon skeletons.
CYCLOPROPANATION REACTIONS
Formation
of two single bonds from a carbon atom is also well known for building up
carbon skeletons. In this process, a three-membered ring is formed by reaction
of a difunctional carbon atom with an olefin. Because of the strain of
three-membered rings, their synthesis is not trivial and a small number of
reactions which effectively append three-membered rings to molecules are
important and widely used.
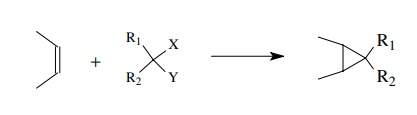
The
Simmons–Smith cyclopropanation utilizes methylene diiodide and a zinc–copper
couple to produce a carbenoid intermediate. This intermediate reacts with
olefins to give cyclopropanes. The geometry of the double bond is preserved in
the cyclopropane.
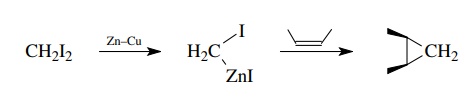
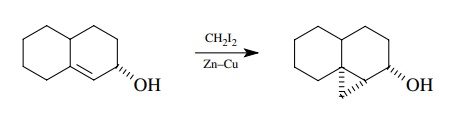
This
addition is sensitive to steric biases in the olefin, and the methylene group
will enter from the least hindered side of the molecule. Alcohol substituents
in the olefin will facilitate the reaction and guide the methylene group syn to
the alcohol.
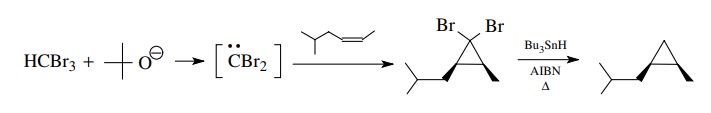
The
base-promoted α eliminations of chloroform
or bromoform provide a simple method for the production of dihalocarbenes.
These add readily to olefinic double bonds to give 1,1-dihalocyclopropanes. The
halogens can be removed (one or both) by reduction; the most common method is
to use tri-n-butyltin hydride.
Another
common method for forming cyclopropanes is to react α-diazoketones or esters with olefins under the influence of copper
or, better yet, rhodium or ruthenium catalysis. Again a metal carbenoid
intermediate is produced which reacts with the olefin.
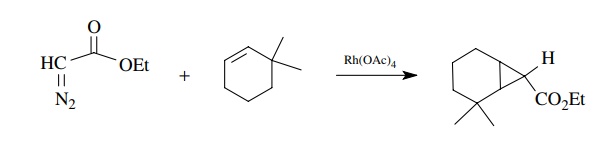
The
importance of this strategy is that functionalized cyclopropanes are pro-duced
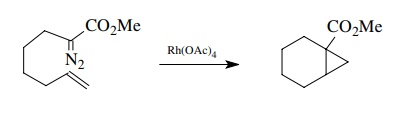
which can be further manipulated. The process can also be carried out
intramolecularly with high efficiency.
Related Topics
