Free-Radical Initiation
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation By Free-Radical Reactions
Besides new insight into the reactivity of free radicals, methods for the production of carbon-centered free radicals have also seen major improvements in the last several years.
FREE-RADICAL INITIATION
Besides
new insight into the reactivity of free radicals, methods for the production of
carbon-centered free radicals have also seen major improvements in the last
several years. One very common new method is to use tin-based reagents as
radi-cal chain carriers. Trialkyltin radicals readily abstract bromine or
iodine from car-bon to produce a carbon-centered free radical. Placement of a
bromide or iodide substituent on a substrate thus permits formation of a
carbon-centered free radical at that position using tin-based methodology. This
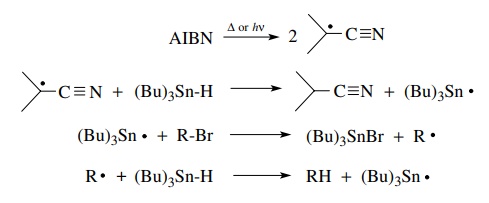
process was initially developed for the reduction of alkyl halides, and it
remains an excellent synthetic method for that purpose. The complete chain
mechanism for the reduction is shown.
One
equivalent of tributyltin hydride is required to reduce one equivalent of alkyl
halide. Each step in the sequence is energetically favored so that side reactions
are minimized. That is, the tributyltin radical does not abstract hydrogen from
the alkyl; it only attacks the halide (Br or I).
Likewise
the carbon radical abstracts the hydrogen from tin much more readily than other
C–H hydrogens. Thus the process is clean from a mechanistic point of view and
consequently leads to clean reduction products.
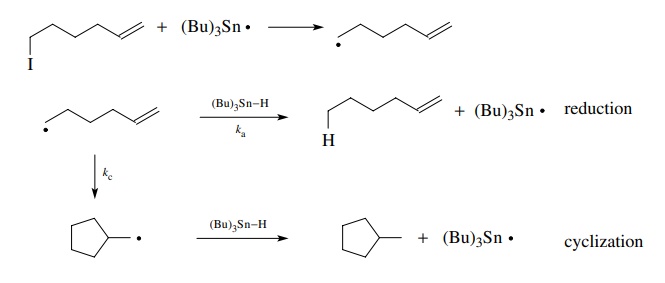
If
this method of free-radical generation is applied to an unsaturated alkyl
halide, the free radical has two pathways available. It can abstract a hydrogen
from tributyltin hydride and give a reduced product as in the reduction process
described above. Alternatively, it can cyclize to give a new cyclic radical
which can abstract hydrogen from tributyltin hydride to give a cyclized product.
These competing propagation steps are shown.
Now
the ratio of products obtained depends on the competing rates of the two
processes. The rate of reduction of the radical is given by ka[R●] [Bu3SnH]
while the rate of cyclization is given by kc[R●].
As was mentioned previously, it has been found that kc is sufficiently large that cyclization is often the
major process. However, reduction is always possible. One way to control
reduction is to note that it is a second-order process which depends on both
the concentration of the radical R● and tributyltin hydride. Thus the rate of
reduction can be lowered simply by running the reaction under dilute
conditions. As the concentration goes down, the rate of reduction goes down
much faster than the rate of cyclization, and cyclization is favored.
After
cyclization has taken place, the only reaction available to the cyclopentyl
methyl radical is hydrogen abstraction. Thus it remains in solution until it
reacts with tributyltin hydride to produce methylcyclopentane and a tributyltin
radical which continues the chain.
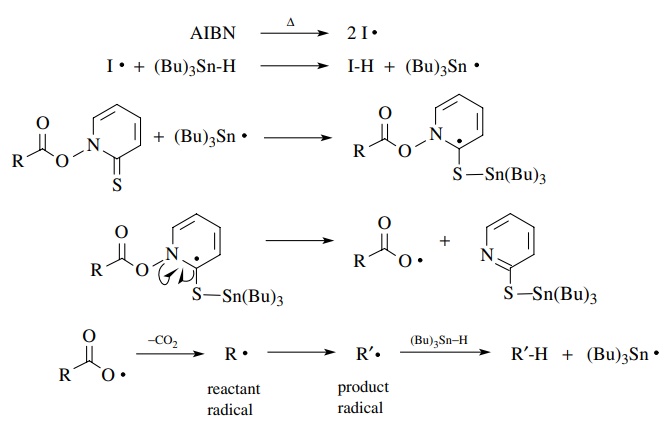
A
second common way to produce free radicals for use in carbon–carbon bond-making
reactions is to use esters of N
-hydroxypyridine-2-thione. This meth-od is also tin based and relies on the
propensity for tin radicals to add to carbon–sulfur double bonds. Subsequent
fragmentation reactions lead to free radicals that are ultimately used in the
propagation steps.
The
free radicals R● participate in a product producing a chain process that
regenerates Bu3Sn● and thus continues the chain. While there are
many steps in this overall sequence, it is a chain process. If each step is
exothermic and kinetically favored, then each step will proceed selectively and
the product will be formed efficiently and reproducibly, irrespective of the
number of the steps in the chain. This turns out to be the case, and the above
method is an effective and reliable sequence for initiating free-radical reactions.
It
is also known that once the tributyltin radical adds to the sulfur of N-hydroxypyridine-2-thione, breakage of
the N–O bond and decarboxylation are very fast and probably concerted. They are
driven energetically by for-mation of the very stable carbon dioxide molecule.
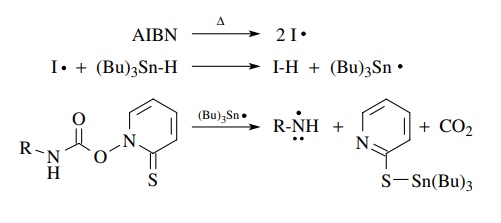
It comes as no surprise that if atoms other than carbon are attached to the
carboxyl group, then they would also end up as free radicals after
decarboxylation. This is shown for a urethane analog as a source of
nitrogen-centered free radicals. A wide variety of other free-radical species
can be produced by this strategy, and it is thus quite useful.
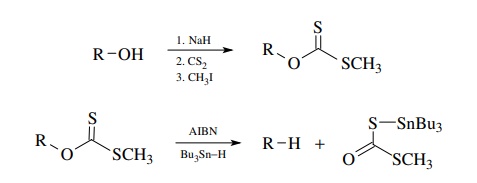
A
third tin-based method of free-radical production also utilizes tin radical
addition to a carbon–sulfur double bond as a key reaction. In this case a
thio-noester (usually a thiono carbonate) is the reactant. As in the previous
method, addition of tin to the sulfur atom is followed by fragmentation to a
carbon-centered radical.
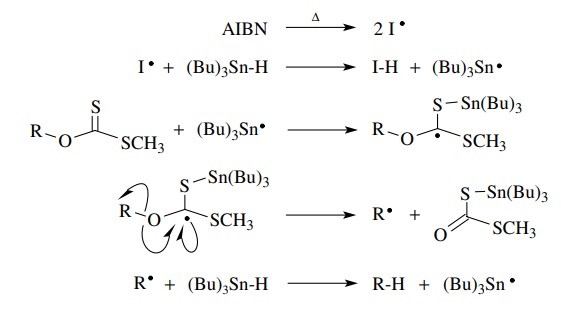
The
driving force for the fragmentation is formation of the C=O double bond. If R● reacts with Bu3SnH,
a tributyltin radical is produced which continues the chain. Carried out in
this manner this reaction is called the Barton deoxygenation of alcohols, since
alcohols are precursors for the thiono esters.
If,
on the other hand, R● is unsaturated and can undergo cyclization rapidly, it
will do so. This competition between reduction of the first formed radical R●
and its cyclization to a new cyclic radical R’● is the same as discussed for
the formation of free radicals from alkyl halides and tributyltin radicals. The
only difference is in the way in which the carbon-centered radical is produced.
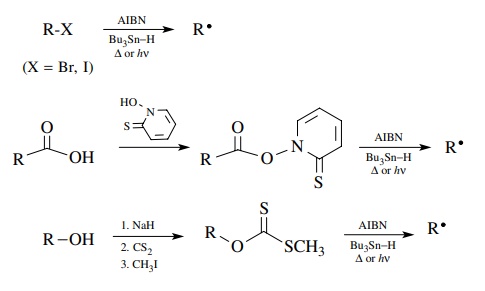
A
number of other strategies have been developed for the production of
free-radical intermediates for carbon–carbon bond construction; however, the
tin-based methods described above are by far the most common. Thus alkyl
halides, carboxylic acids, and alcohols are all excellent precursors for free
radi-cals by these methods. The choice of method can be made on the basis of
which substrate fits into the synthetic scheme most efficiently or which
precursor is most readily available.
Related Topics
