Cloning Vectors - Enabling Techniques
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Recombinant DNA Technology
Genes present in DNA fragments that have been excised with restriction endonucleases can be maintained (replicated) and expressed by inserting them (cloning) into vectors after ligation.
CLONING VECTORS
Genes present in DNA fragments that have been excised with restriction
endonucleases can be maintained (replicated) and expressed by inserting them
(cloning) into vectors after ligation. These cloning vectors are of different
types and are relatively small DNA molecules that have the ability to self-replicate
in a host cell. The main type of vectors used for gene cloning are plasmids,
cosmids and bacteriophages, which are normally used according to the size of
the DNA fragments that need to be cloned.
a)
Cloning of small fragments of DNA
To allow the cloning of small DNA fragments, generally up to 10 kb,
plasmids are the main vectors of choice.
i) Plasmids
Plasmids are circular extrachromosomal DNA molecules that replicate
independently within cells using their own origin of replication. There are a
number of features generally found in plasmids used as cloning vectors:
• Antibiotic resistance markers.
These are genes coding for proteins
that confer resistance to specific antibiotics. These markers therefore allow
the selection of hosts carrying the plasmids.
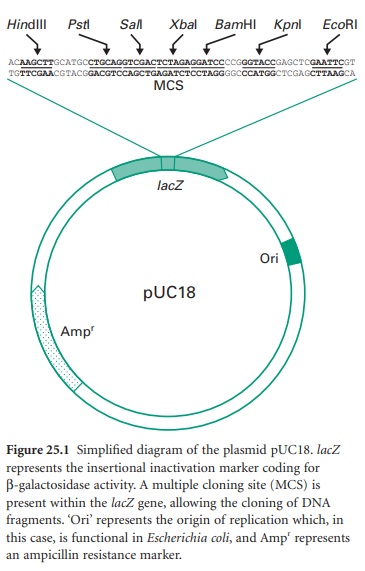
• Multiple cloning sites (MCS).
These are stretches of DNA designed to
contain a number of unique different restriction sites, providing a choice of
possible restriction enzymes to be used for the cloning of DNA fragments.
• Origin of replication.
Required for plasmid replication in a
specific host. These DNA sequences also determine the number of copies at which
the plasmid will replicate, from just one to several hundreds per cell.
Plasmids having more than one host-specific origin of replication are known as
shuttle vectors.
• Insertional inactivation
markers.
These facilitate the selection of
recombinant from non-recombinant plasmids. The most commonly used is the lacZ gene coding for β-galactosidase.This enzyme
cleaves 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), an artificial
substrate that mimics galactose, which results in the generation of an
insoluble blue product. Insertion of a recombinant DNA fragment in lacZ will result in the disruption of
β-galactosidase production and the inability to cleave X-Gal. Hence colonies
from bacteria carrying an intact lacZ gene, i.e.
from non-recombinant plasmids, appear blue on agar plates containing X-Gal. In
contrast, those with a successful insertion in lacZ,
i.e. harbouring recombinant plasmids, appear white.
Figure 25.1 shows
all these features in a simplified diagram of the cloning vector pUC18.
An ideal plasmid for cloning should be small (2–10 kb) and
conjugation-defective, i.e. non-self-mobilizable from cell to cell, and produce
a selectable phenotype in host cells. It should also contain a large MCS and
replicate at a high copy number (>10 copies per cell).
b)
Cloning of large fragments
of DNA
Sometimes there is a need to clone large fragments of DNA, for example
for the isolation of complete gene clusters. An example would be the cloning of
large eukaryotic genes or genes required for the synthesis of a certain
molecule. Sometimes the synthesis of a compound requires more than 10 different
genes and these are frequently organized in operons cotranscribed in a single
mRNA molecule. To enable the cloning of large genes or full-length operons,
vectors such as bacteriophages, cosmids or BACs need to be used.
i) Bacteriophages
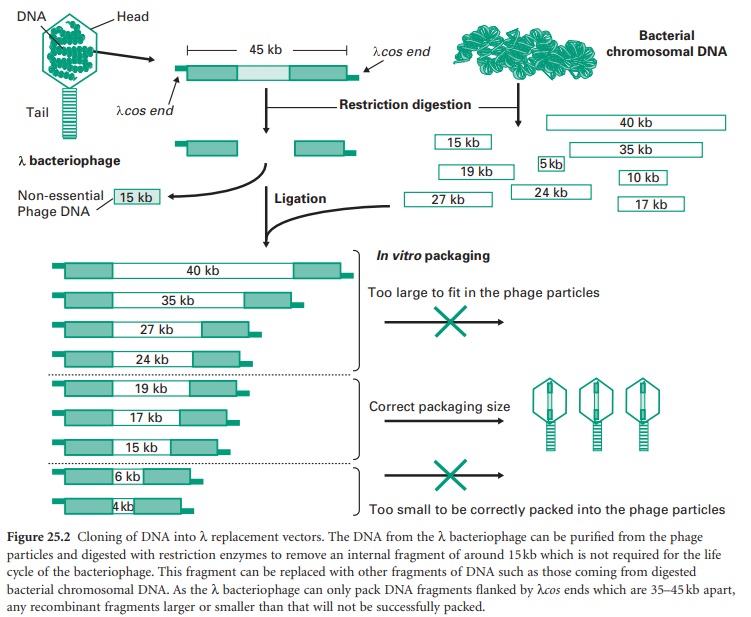
The most popular has been the E. coli λ (lambda) bacteriophage, which is
composed of a tubular protein tail and a protein head packed with approximately
50 kb of double-stranded (ds) DNA (Figure 25.2).
After injection of the viral DNA into E. coli,
bacteriophage λ can multiply and enter a lytic cycle leading to the lysis of
the host cell and the subsequent release of a large number of phage particles.
Alternatively, injection of the DNA can lead to a lysogenic cycle in which the
phage DNA is integrated into the E. coli chromosome
where it is maintained until the environmental conditions change and is then
excised, entering a lytic cycle. Out of the 50 kb that make the λ bacteriophage
less than half are essential for its propagation and therefore around 20 kb can
be replaced by recombinant DNA, hence their name λ replacement vectors. For this reason these are very
useful vectors for the generation of genomic libraries. DNA in λ bacteriophages
contain at each end small single-stranded (ss) complementary DNA fragments
called λ cohesive ends (λcos ends).
Recombinant phages can be assembled in a test tube into phage-like particles by
enzymes which recognize and process the λcos ends,
provided that they are 35–45 kb apart. These enzymes as well as the head and
tails required for the assembly process are commercially available as part
of in vitro packaging kits. The in vitro packaging results in the formation of
recombinant phages that can be transduced to E. coli cells.
Transduced E. coli will be identified by
the formation of lysis plaques in agar plates seeded with a mixture of E. coli and recombinant λ bacteriophages.
ii) Cosmids and fosmids
Cosmids are plasmids maintained
in E. coli that have been engineered to carry λcos sequences. This allows their packaging in vitro into λ phage particles to be transduced
into E. coli cells for replication. Once inside the
bacterial host the cosmid DNA is circularized by the joining of the λcos ends and thereafter it replicates as a normal
plasmid. This implies that the cloned DNA will be available as E. coli colonies and not as plaques like with the
λ bacteriophages. As less DNA is required for plasmid than for bacteriophage
replication, cosmids can carry up to 40 kb of cloned DNA still enabling the
packaging into λ phage particles.
Eukaryotic and more particularly
mammalian genomic DNA rich in multiple repeated elements can be subject to
deletions and rearrangements when cloned in standard multicopy cosmids and
replicated in bacteria. This inconvenient can be reduced by using special
cosmids in which the multicopy origin of replication has been replaced by the
single-copy origin of replication from the E. coli F′
factor. These cloning vectors are known as fosmids.
iii) Bacterial artificial chromosomes
Although cosmids and fosmids enable the
cloning of relatively large DNA fragments, the amount of DNA that can be packed
into a λ bacteriophage head is limited to around 50 kb. To clone even larger
DNA fragments cloning vectors derived from the single-copy E. coli F′ factor, similar to the fosmids, have
been designed. In this case the λcos ends are
absent or not used for packaging and instead the recombinant plasmids are
introduced directly into the E. coli cells
by transformation. This allows the cloning of DNA fragments of several hundreds
of kilobases resulting in what are then called bacterial
artificial chromosomes(BACs).
Related Topics
